

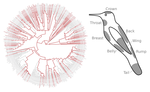
Ecology needs rules stipulating how species distributions and ecological communities should be assembled along environmental gradients, but few rules have yet emerged in the ecological literature. The search of ecogeographical rules governing the spatial variation of birds colours has recently known an upsurge of interest in the litterature [1]. Most studies have, however, looked at pigmentary colours and not structural colours (e.g. iridescence), although it is know that color perception by animals (both birds and their predators) can be strongly influenced by light diffraction causing iridescence patterns on feathers.
In the present study [2], the authors study ca. 190 ecological communities of hummingbirds as a function of their iridescent colors, in a large study zone spanning varied habitats across Ecuador. They show that colour composition of local hummingbirds communities are shaped by two main processes :
(i) phenotyping clustering of birds with similar dorsal colours, due to local selection of species with similar camouflages against predators (i.e. some sort of mimetic circles).
(ii) phenotypic overdispersion of birds with distinct facial and ventral colours, resulting from character displacement and limiting reproductive interference.
I found this second result particularly interesting because it adds to the mounting evidence that character displacement (also for songs or olfactory signaling) allow local coexistence between closely-related bird species once they have reached secondary sympatry. It is important to note that not all color patches though to be involved in sexual selection followed this overdispersion rule -- throat and crown color patches were not found overdispersed. This suggests that further investigation is needed to determine how color variation shape the structure of hummingbird communities, or bird communities in general.
Another notable quality of the present study is that it is making extensive use of museum specimens and thus shows that very innovative research can be performed with museum collections.
References
[1] Delhey, K. (2019). A review of Gloger’s rule, an ecogeographical rule of colour: definitions, interpretations and evidence. Biological Reviews, 94(4), 1294–1316. doi: 10.1111/brv.12503
[2] Gruson, H., Elias, M., Parra, J. L., Andraud, C., Berthier, S., Doutrelant, C., & Gomez, D. (2019). Distribution of iridescent colours in hummingbird communities results from the interplay between selection for camouflage and communication. BioRxiv, 586362, v5 peer-reviewed and recommended by PCI Evolutionary Biology. doi: 10.1101/586362
DOI or URL of the preprint: https://doi.org/10.1101/586362
Version of the preprint: 1
Dear authors,
Both reviewers see significant improvements in your manuscript.
I also think there is merit in your work and that it could be considered for recommendation after another round of revisions.
Indeed, one reviewer raises a number of significant issues that deserve further consideration. These concerns :
the lack of intraspecific sampling, which can be problematic for a phylogenetic study. I understand you might not be able to fix this issue by increasing sample size in all study species, but may be you do have some data (even on very few species) allowing to gauge to what extent there is no problem with the intraspecific variation. I guess you performed some kind of repeatability test of this kind on at least a few species of your study group.
the question of null model testing and whether the used null model is appropriate for the study hypotheses. In particular is it possible to detect phenotypic overdispersion in certain traits ? Null models must be applied carefully so you need to provide the rationale that they are suited to test your working hypotheses. This can be fixed by elaborating on this rationale in the method section for instance, and perhaps later discussing these methodological choices.
extrapolation of processes based on pattern analyses. This can be fixed in the discussion, by toning down some conclusions, or at least acknowledging that distinct processes may produce similar patterns. Also, empirical literature can sometimes be used to make a point about the process that is most likely acting. E.g. is there any convincing data suggesting the role of color patterns in hummingbirds relative to camouflage from predators ?
I hope this helps improving the manuscript again.
Best regards
Additional requirements of the managing board:
Please ignore this message if you already took there requirements into consideration.
As indicated in the 'How does it work?’ section and in the code of conduct, please make sure that:
-Data are available to readers, either in the text or through an open data repository such as Zenodo (free), Dryad (to pay) or some other institutional repository. Data must be reusable, thus metadata or accompanying text must carefully describe the data.
-Details on quantitative analyses (e.g., data treatment and statistical scripts in R, bioinformatic pipeline scripts, etc.) and details concerning simulations (scripts, codes) are available to readers in the text, as appendices, or through an open data repository, such as Zenodo, Dryad or some other institutional repository. The scripts or codes must be carefully described so that they can be reused.
-Details on experimental procedures are available to readers in the text or as appendices.
-Authors have no financial conflict of interest relating to the article. The article must contain a "Conflict of interest disclosure" paragraph before the reference section containing this sentence: "The authors of this preprint declare that they have no financial conflict of interest with the content of this article." If appropriate, this disclosure may be completed by a sentence indicating that some of the authors are PCI recommenders: “XXX is one of the PCI XXX recommenders.”
I have carefully read the revised version of the manuscript "Distribution of iridescent colours in hummingbird communities results from the interplay between selection for camouflage and communication". I provided comments on an earlier version, and although I see notable improvements, I still have some serious concerns about the approach taken and the conclusions that are drawn from the results. I hope the authors find these useful.
Firstly, measurements from one single individual per species seriously reduce the confidence one has on the degree to which the color measures will be representative of the species. Furthermore, it is not clear from the Methods whether the selected individual was from the communities which are being compared, as there is no way to discard - at least minor - geographic variation in coloration along the specie's range. Comparative analyses across a number of species rely on precise and representative estimates of species' average trait values, and this becomes seriously undermined when one individual per species is measured. The author's argument that they selected an individual whose coloration was representative of other specimens available in the collection is undermined by another argument, in the ms itself, in favour of the use of spectrophotometry for color measurement as being superior to human visual estimates. Skeptical readers would be allayed if estimates of the amount of within vs among species variation were provided.
Secondly, I am concerned about the null models used to determine over dispersion vs clustering. The null models represent community compositions based on random sampling from the whole set of species included in the study. This is fine. However, when looking at the trait distribution along the phylogeny it is clear that there are quite notable differences in the degree of color variation among the patches that were measured. The back, for example, is almost always green(ish) to green-brown, with the odd cream coloured or purple species (two in the whole set), while the throat coloration (as is well known for hummingbirds) spans a much wider gamut of colors. What this means is that at least phenotypically, it is much more difficult (not to say virtually impossible) for over-dispersion to be detected for the back as there is virtually no variation in coloration in that patch in the set of studied species. On the other hand, it is similarly difficult to detect clumping for the coloration of the throat as there is a very large amount of variation. In other words, given the large differences in phenotypic variation between patches, the null models appear to "stack the deck" in favour of a particular hypothesis.
Thirdly, assuming we trust the results, these provide information about a pattern: clustering or over-dispersion of species in a community, based on phylogeny or for a given patch-coloration. However, the authors make a leap-of-faith to extrapolate a process from the observed patterns, that clustering suggests that similar coloration results from selection to avoid predators, while over-dispersion occurs in patches that play a role in species recognition. These are but two of potentially many different explanations for the observed patterns. Revell et al. (2008) have warned against interpreting evolutionary processes responsible for observed presence or absence of phylogenetic signal in a given trait, and their arguments apply as well for cases such as this one. The results provide information on a pattern, the evolutionary process that has given rise to said pattern cannot be discerned, at least not with the data that is available to the authors in the present manuscript. For example, no evidence is provided for the role of different patches in camouflage from predators or intraspecific recognition.
I have one minor further note about the implementation of one of my suggestions. The conclusion that character displacement acts on certain patches while other patches are under selection for crypsis is well supported by the addition of supplementary figure 4, which shows the hue of the 8 main patches, and on line 325, you say “co-occurring hummingbird species tend to display the same hues on dorsal patches.” It is clear from supplementary figure 4 that back and wing colors are conserved as brown and green specifically across the phylogeny, but you do not explicitly say so in the text of the manuscript and the authors do not refer to figure S4 in the manuscript body at all. I think that these specific colors are key support for the crypsis hypothesis and including this information in the main text might strengthen your argument.
Otherwise, the authors have fully addressed my previous recommendations as well as those of the other reviewer. I think that the changes to the hummingbird outline figure render the clear, readable, and compelling. Including the hypotheses and predictions as a main body table has certainly improved the readability and flow of the manuscript. I would therefore recommend this paper pending further revisions from other referees.
https://doi.org/10.24072/pci.evolbiol.100199.rev22DOI or URL of the preprint: https://doi.org/10.1101/586362
First, I would like to apologize this overly long delay for taking an editorial decision. As I found this study quite intriguing, I wanted to take the time necessary to take a careful decision about it.
Both reviewers found your study interesting and quite novel, and raised no substantial issue on how the study is executed. I fully agree with them. Nevertheless, they expressed a number of concerns about how the rationale of the study is being explained, how the theoretical and empirical background of the study are elaborated (mainly in the introduction), and how some interpretations are drawn from the results. At this stage, it is important to take all reviewers' comments into account in order to make this paper have the impact it deserves.
I think that your work explores novel questions in evolutionary community ecology and will contribute to opening up a new field of research. Therefore it deserves further consideration and very likely publication.
Additional requirements of the managing board:
As indicated in the 'How does it work?’ section and in the code of conduct, please make sure that:
-Data are available to readers, either in the text or through an open data repository such as Zenodo (free), Dryad (to pay) or some other institutional repository. Data must be reusable, thus metadata or accompanying text must carefully describe the data.
-Details on quantitative analyses (e.g., data treatment and statistical scripts in R, bioinformatic pipeline scripts, etc.) and details concerning simulations (scripts, codes) are available to readers in the text, as appendices, or through an open data repository, such as Zenodo, Dryad or some other institutional repository. The scripts or codes must be carefully described so that they can be reused.
-Details on experimental procedures are available to readers in the text or as appendices.
-Authors have no financial conflict of interest relating to the article. The article must contain a "Conflict of interest disclosure" paragraph before the reference section containing this sentence: "The authors of this preprint declare that they have no financial conflict of interest with the content of this article." If appropriate, this disclosure may be completed by a sentence indicating that some of the authors are PCI recommenders: “XXX is one of the PCI XXX recommenders.”
I have carefully read the pre-print "Distribution of iridescent colours in hummingbird communities results from the interplay between selection for camouflage and communication". I was intrigued by this work which addresses an interesting question in a charismatic group of birds. The manuscript is generally well written, however I do have issues with how the hypotheses are presented, how some of the analyses are done and the conclusions that are drawn from the results.
Major comments:
In the introduction the rationale and theory behind the predictions is not at all clear, which makes it very hard to understand what supports them, and also the results and importantly conclusions that are drawn from the results. An example is ln 57-59: where it is stated that co-occurring species are expected to converge in coloration because of predation risk. There are many implicit "all else being equal" here that should be made explicit. Also, it is not stated why different patches might be under different selection pressures and this seems highly important. What evidence is there for some colors in hummingbirds being related more to crypsis, while others might be more associated with species recognition? I would think this is key to the hypothesis that different patches would present different patterns of dispersion within a community. This refers to the predictions presented in lns 86-89. There is no theoretical support presented for these predictions and the lack of such theory buttressing the predictions makes the author's rationale hard to follow.
In ln 65-66 the authors state that "misidentification can also lead to misdirected aggression and costly fighting when individuals compete for resources or territories". I am not an expert in hummingbird behaviour, but I think that inter-specific aggression is somewhat common in this group, there are descriptions of dominance structures between different species based on size.
Ln 79: the authors state that previous studies relied on human vision, which is likely to have biased the results. It is not stated how or why. In addition recent work suggests that for some purposes human vision can detect much of the variation in coloration in the visible range (see e.g. Bergeron and Fuller 2018, Dale et al 2015).
In Ln 85: The authors introduce the model system but it comes a bit out of the blue, without any justification to why hummingbirds are a good system. This only comes up in the Methods.
Ln 136: a single male was measured for each species. This is worrisome as no information is provided on how said male was chosen, nor on the degree of within species variation as opposed to among species variation. Readers might worry to what degree a single male can be representative of the whole species. Also related to this, were several measures taken of the same patch? What was the repeatability among measurements for the same individual and patch? Do patches vary in the repeatability?
Ln 194-195: random assemblages from a species pool containing all species from all communities were used as null models. It would be good to justify the choice of null model and make explicit what such null model entails, from an evolutionary or ecological point of view. The choice of null model is likely to have an important influence on the results.
Ln 200-201: analyses are undertaken for all patches together, i.e. creating a color volume per species (if I understood correctly), and then repeated for each patch independently. The latter increases the number of analyses, and there is no justification for repeating the analyses for each patch other than the fact that the whole-species color volume might not capture subtleties of particular patches. Why not run a preliminary analysis to see whether some patches tend to present similar colours repeatedly across all species? This would enable the authors to justify grouping some patches and analysing others separately.
Ln 204: the authors use a subsample of phylogenies for the group of interest from birdtree.org Why did they prefer to use the trees from birdtree.org rather than the McGuire et al 2014 phylogeny?
Ln 239-242: I don't understand the rationale behind "removing" the effect of shared ancestry and then analysing clustering or overdispersion in coloration. The authors already found that species are composed more commonly by closely related species, so I wonder whether it actually makes sense to even attempt to "remove" the effects of shared ancestry. Also, it is unclear what exactly the authors are doing here. What exactly is being "removed"? The fact that the results change notably between the analyses does lead one to wonder whether it is a statistical artefact or whether these results reflect a biological pattern of interest.
Ln 253-255: the authors state that results suggest there is a trade-off between selection for camouflage and species recognition. However, I do not see which results suggest there is any trade-off, nor which results suggest some coloration is used for camouflage and which is used for species recognition. This is partly due to the fact that the theory behind the hypothesis and predictions has not been presented to the readers, so we cannot draw the same conclusions as the authors do.
Ln 284-285: the authors state that a previous work, on the same dataset, but using different methods, found similar results. But the authors do not contrast their results with those of the previous work, nor enlighten the readers as to whether the different methodology might impact the results, how or why.
Minor suggestions:
ln. 18 and ln 54-55: the authors state that co-ocurring species that share the same environment would be expected to have similar appearances due to selection for crypsis. However, merely overlapping in the distribution is likely insufficient for selection on predator avoidance to lead to similar coloration. Surely there are other factors that are hugely important beyond the environment. For example, size of the prey will have an important impact on predation risk, habits (diurnal vs nocturnal) will also have an important influence, as will behaviours, and other factors.
ln 31. It is unclear why it is concluded that over dispersion observed in some patches and clustering on others suggests one may counter-balance the effect of character displacement. If different patches respond to different selection pressures, i.e. colors in some patches are signals for species recognition, while other patches function for crypsis, no counter-balance is expected, or?
ln 60 (and elsewhere): "species assortment locally", I guess you mean local species sorting.
ln 72: worth noticing, change to worth noting.
ln 100: replage large with long.
ln 260: As predcited in our prediction 5, redraft.
https://doi.org/10.24072/pci.evolbiol.100199.rev11This manuscript investigates the community-level and phylogenetic distribution of iridescent colors in hummingbird communities in Ecuador. The authors use spectrometry data and a set of field observations to examine the distribution of different color variables within and between communities and to elucidate which variables of color change at variable phylogenetic and community scales. The topic is fairly original in that it attempts to examine color macroevolution in an ecological framework, aided by the fact that the study system contains many co-occurring taxa with varying degrees of relatedness which have diversified using an understudied and complex plumage coloration mechanism. This is an informative and novel study and the reviewer therefore recommends that the authors revise the manuscript to reflect the enclosed suggestions. The conclusion that character displacement acts on certain patches and certain plumage color axes makes intuitive sense and is supported by the evidence presented. However, the conclusion which assumes that co-occurrence of similar phenotypes represents convergence or environmental filtering due to camouflage may be aided by some description or quantification of the colors which are exhibited in the differing light environments of the canopy and understory. For the most part this paper is clear and readable. The most confusing aspects of the paper involve the use of jargon specific to methodology. There is a clear distinction made between phenotypic and phylogenetic dispersion, but when these terms are used in close proximity it is sometimes hard to mentally track these terms and the matrix of hypotheses. It potentially might be clearer to refer to biological hypotheses in plain text as opposed to numerically or to include table S2 as a main-body table. Additionally, it would be nice to have some description of the actual colors which are on these birds. If colors on the back are being conserved are these mostly brown or gray patches? When they vary within a community what are the color axes of the variation?
Line-By-Line Comments
130: Interesting approach to look at environmental organization data for hummingbird assemblages, I’d wonder if these accounts are biased and perhaps may not provide a clear picture of co-occurrence, especially for rarer taxa.
196: Good justification for the patch-specific analysis.
100: This is an interesting prediction. I do wonder if as a human the hue shift effect between close and long-distance is visible and if there would be a way to model the distance-based effect. Or is it more a function of the angle?
126: For the species which coexist, is hybridization common? How closely related are the “closely related” co-occurring species? Are they con-generics? Overlapping subspecies?
141: How did you measure homology of these “extra” patches. If they were present in multiple taxa why didn’t you include them as key patches while circling patches?
169: Remove “called” here.
175: Was a shearwater the closest related visual-model taxon you could find? This ecologically seems like an odd choice.
176:: Are you referring here to the actual illuminant you used or a habitat-simulation model you used to transform your data? If so, wouldn’t this prevent you from performing meaningful standardized environmental comparisons? It might be good to find a way to compare the canopy and understory birds which co-occur as that could be an important source of variance.
Table 1: I like the color scheme here and find the data presentation intuitive but the pluses and minuses may be a little bit hard to see. It may be helpful also to include a statement about the point you’re trying to show with this table. For example, are you trying to show the difference between Phenotypic structure and decoupled phenotypic structure? Between the different variables? What patterns can be illuminated to guide the reader? Also, which aspects of hue best describe the clustering (eg, light to dark, brown to gray)? Do the three variables which describe hue have similar variance or is variance more distributed along certain hue axes?
253: The suggestion that colour structure results in a camouflage-dependent tradeoff depends on the assumption that the environmental clustering represents selection on camouflage, but these patterns could be driven by mimicry as in woodpeckers or environmental adaptive gradients such as gloger’s rule that may not be driven by crypsis. One powerful way you could fix this is by comparing the canopy and understory birds to see if color differences in patches supposedly used for crypsis are best explained by habitat.
290: Low dispersal ability as compared to what? Also in reference to line 285 could this result be biased by the selection of sample sites in contact zones? Just because these birds co-occur now in some parts of their range doesn’t mean that their plumage didn’t evolve in allopatry. Testing evolutionary models for each patch may give you a finer grained approach to understand the evolutionary history of the integrated phenotypes.
292: I’m still not sure I understand why this pattern would indicate filtering on another trait and not ?
302-314: It would be helpful to mention some of the elaborate behavioral adaptations that hummingbirds use to take advantage of iridescent gorget’s for instance. [CITATION]
309: Once again, you have the canopy/understory data, why don’t you compare the effect of these two habitats? That would be a really compelling test or an interesting note if you tested this and it didn’t matter. Would you see the same pattern if you only tested understory or canopy birds?
332: It is stated that hue shift may be less variable to predators but wouldn’t any iridescence on the wings and back have a flashing reflective effect, attracting attention?
335: The allusion to variable layer structure seems speculative, you should note that nano-scale imaging of the layer structure would be necessary to know if the layer variation you allude to is real.
336: Once again, the numbering of predictions is difficult to follow and the frequency with which you refer to them suggests that figure S2 is more of a key guide for the reader than its placement in the supplement would suggest.
359: How conserved are these patch colors across the phylogeny? Do they represent perhaps a historically conserved patch? Once again, evolutionary model fits may elucidate these historical questions.
https://doi.org/10.24072/pci.evolbiol.100199.rev12