

 based on reviews by Ray Blanchard and 1 anonymous reviewer
based on reviews by Ray Blanchard and 1 anonymous reviewer
Natural populations do not consist of only perfectly adapted individuals. If they did, of course, there would be no fodder for evolution by natural selection. And natural selection is operating all the time, winnowing out less well adapted phenotypes through differential reproduction and survival. Demonstrations of natural selection modifying characters-state distributions to bring phenotypes closer to their optima abound in the evolution literature, with examples of short- and long-term changes in phenotype and allele frequencies.
However, evolutionary biologists know that populations cannot reach their adaptive peaks. Natural selection is tracking a moving target, always with some generations of lag time. The adaptive landscape is multidimensional, so the optimal combination of multiple character states may be impossible because of constraints and trade-offs. Natural selection does not operate alone or in isolation – new mutations and migrants that were selected under other conditions will inject locally non-adaptive genetic variation and genetic drift can change allele frequencies in random directions. We understand these processes that generate and maintain less advantageous variants on a continuous gradient from an optimal phenotype in a fitness landscape. More puzzling are heritable polymorphisms with distinct morphologies, physiologies or behaviours maintained in populations despite their measurably lower reproductive success. But a complete model of evolution must also be able to accommodate these Darwinian paradoxes.
Raymond et al. (2023) investigate one such Darwinian paradox: In humans, male homosexual mate preference is heritable and is associated with a large reduction in offspring production but nonetheless occurs at relatively high frequencies in most human populations. Furthermore, multiple studies have found that homosexual men come from families that are, on average, larger than those of heterosexual men and that homosexual men have, on average, higher birth rank than do heterosexual men, i.e., having more older siblings and, particularly, more older brothers. Two types of mechanisms consistent with these observations have been proposed: 1) An evolutionary mechanism of sex-antagonistic pleiotropy, whereby highly fecund mothers are more likely to produce homosexual sons, and 2) A mechanistic explanation whereby successive male pregnancies alter the uterine environment by increasing the probability of an immune reaction by the mother to her male fetus, altering development of sexually dimorphic brain structures relevant to sexual orientation.
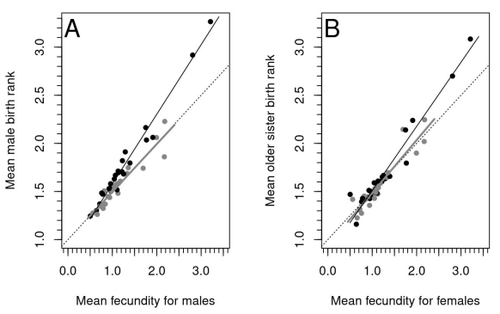
In this article, the authors explore these two mechanisms of sex-antagonistic effects (AE) and fraternal birth order effects (FBOE) and test how well they account for patterns of male homosexuality in population and family data. Clearly, these two effects are somewhat confounded because high birth ranks can only occur in large families. If, indeed, the probability of male homosexuality increases with increasing numbers of (maternal) older brothers, homosexual males will be more common in larger families. Similarly, if high female fecundity leads to a higher probability of male homosexuality via sex-antagonistic effects, homosexual males will, on average, have more older brothers. To disentangle the actions of these two effects the authors modelled the relationship between birth rank and population fecundity and investigated whether AE or FBOE modified this relationship for homosexual men. Simulation results were compared with aggregated population data from 13 countries. Family data on individuals’ sexual preference, birth rank and number of male and female siblings from France, Greece and Indonesia were analysed with generalised linear models and Bayesian approaches to test for a signal of AE or FBOE.
These analyses revealed a significant older-brother effect (FBOE) explaining patterns of occurrence of homosexuality in population and family data but no significant independent sex-antagonistic effect (AE). Thus larger family sizes of homosexual men appear due to the older-brother effect, with individuals of high birth rank coming necessarily from large sibships. The simulation approach also revealed that modelling a fraternal birth order effect (FBOE), such that individuals with more older brothers are more likely to be homosexual, generates an artefactual older sister effect simply because homosexual men are overrepresented at higher birth ranks. Older-sister effects reported in the literature may, therefore, be statistical artefacts of an underlying older-brother effect.
This paper is interesting for a number of reasons. It does an excellent job of explaining, identifying and dealing with estimation biases and testing for artefactual relationships generated by collinearity. It applies state-of-the art analytical/statistical tools. It breaks down two colinear effects and shows that only one really explains phenotypic variation. This is a great example of how to disentangle correlated variables that may or may not both contribute to trait variation. But most intriguingly, we are left without evidence for an evolutionary mechanism that compensates the large fitness cost associated with male homosexuality in humans. How can we explain high heritability maintained in the face of strong directional selection that should erode heritable genetic variation? The usual suspects include cryptic compensatory mechanisms yet to be discovered or flawed estimates of selection or heritability. For example, data on heritability of male homosexual mate preference in humans come from twin studies and twins share birth rank as well as alleles. Thus it is possible that heritability is over-estimated, including the environmental component associated with birth rank.
If, as the authors demonstrate here, birth rank is the strongest predictor of male homosexual mate preference, selection may be acting on a non-heritable plastic component of phenotypic variation. This could explain why heritable variation is not exhausted by selection, rendering the paradox less paradoxical, but fails to provide an adaptive explanation for the maintenance of male homosexual mate preference.
References
Raymond M., Turek D., Durand V., Nila S., Suryobroto B., Vadez J., Barthes J., Apostolou M. and Crochet P.-A. (2023) Increased birth rank of homosexual males: disentangling the older brother effect and sexual antagonism hypothesis. bioRxiv, 2022.02.22.481477, ver. 4 peer-reviewed and recommended by Peer Community in Evolutionary Biology. https://doi.org/10.1101/2022.02.22.481477
DOI or URL of the preprint: https://doi.org/10.1101/2022.02.22.481477
Version of the preprint: 3
All small suggestions and English improvements have been incorporated. Answers to more developed comments (in bold) are detailed below.
Table S2. I do not think that p0 (this is the probability that the firstborn is homosexual, no? and it a constant 0.05) increases with birth order but rather that p (i.e., the probability that a sampled individual is homosexual) increases with his birth order.
Thanks for pointing out this typo. Yes, when FBOE is modelled p0 is not affected, and when AE is modelled, p0 is increased. Legend of Table S2 has been edited to clarify.
Table S2. Is this technically a "slope"? This is modelled as a threshold, no?
Yes, it is a slope. Whatever the function used to model FBOE (including a function with a threshold), mean birth rank and mean fertility could be described by a line. This is shown, for example, in figure S3
 , posted 24 Nov 2022, validated 25 Nov 2022
, posted 24 Nov 2022, validated 25 Nov 2022Dear Michel,
Thank you for this revised version and sorry for the time to review it. Everything looks good to me, but I still have a series of remarks/questions - on the main text and on the supplementary material - which are indicated in the attached file.
Thanks in advance for the answers to these last questions/comments.
Best regards,
Jacqui Shykoff
DOI or URL of the preprint: https://doi.org/10.1101/2022.02.22.481477
Version of the preprint: v2
 , posted 22 Sep 2022
, posted 22 Sep 2022Dear All,
I appologise for the time spent on this.
First, the new version of the manuscript is far clearer and it definitely merits recommendation, but there are some things that are not as clear as they should be.
The manuscript needs a bit of tidying.
In some places you refer to the wrong figure in the text.
Some problems are just minor wording details and I have annotated, I think, everything that I feel should be changed. However occasionally the wording problems can also lead to confusion. Please go through this carefullly and make sure that it is explicitly clear when you are refering to data and analyses of data and when you are refering to your simulations and that the difference between the aggregated and the individual family data is clear. I was confused in a few spots as to what was derived or calculated from simulations and what was infered from analyses of data.
Organisational comment: You make methodological developments that are not actually mentioned in the Methods section. Perhaps that is not a problem, but when you present the results of these methodological developments in the Results section a lot of it is actually "methods". Also the Results of the data analyses that you present in the Methods section arrive at the end of the Results section ... and I wonder if it would not be clearer to reorganise somewhat so that we can find the structure of the Methods in the Results and vice versa.
I have highlighted and commented where I felt the methods or presentation were confusing. This includes some of the descriptions of your models and presentation of your results. I am not sure that my suggestions are always correct but this points out that your text was not adequately limpid.
I will write my recommendation now because this manuscript just needs some clarification. I think the conclusions are all sound and will not change.
I hope you can see all my comments on the attached documents. I seem unable to attach two document. I will send the documents separately.
Download recommender's annotationsDOI or URL of the preprint: https://doi.org/10.1101/2022.02.22.481477
Dear Recommender,
Thanks for all the comments, from you and the two reviewers. All were very helpful and contributed greatly to improved the paper. Please find below specific answers to each of them. When a page number is indicated, it corresponds to the version without track changes.
Sincerely yours,
Michel Raymond
 , posted 24 Apr 2022
, posted 24 Apr 2022Dear Authors,
Your very interesting preprint has now been read and assessed by two independent peers who both point out a lot of merit and value in the work. However, both suggest a number of changes that would make this work stronger, clearer and more valuable to the scientific community.
Your reviewers have done an excellent job and provide several useful, constructive suggestions. I have also annotated a copy of your preprint with highlighting and comments that I hope will be easily interpretable. In many places I suggest alternative wording but I also pose a number of questions and point out what I perceive to be inconsistencies. If there is any problem for you in figuring out what I am asking you to do I will provide you with a numbered list, though the easiest for me would be if you could provide me with an editable text in open office or word.
I note that both reviewers suggest an independent assessment of the statistical methods by an expert, and reviewer 2 pleads for more clarity and transparency in your presentation of the modelling and analyses. Because I have already spent far too long before getting back to you with a decision (we just moved our lab and there have been a number of things keeping me busy) I wanted to get this work back into your hands for a thorough revision of the text and presentation. At the same time, I will look for an appropriate expert in these analytical methods to provide you with more precise suggestions on how to present that part of the manuscript with better clarity. Already, I think, the comments and suggestions of the two reviewers will guide you in improving all aspects of this work including the model and the analytical methods.
I add here a final comment to accompany the notes in the attached file:
The statstical/analytical problem you set out to ellucidate seems to me to be an example of a not uncommon problem of colinearity. A Fraternal Birth Order Effect that will be increasingly visible in large sibships and a maternal fertility effect will, on average, generate a Fraternal Birth Order Effect because you will only find males of high fraternal birth order in large sibships. Now I recognise that introducing additional specific terminology (can we call this jargon) does not usually increase clarity or enhance understanding, but colinearity is a common problem since explanatory variables are often correlated in observational data (size often increases with age, ecological and phylogenetic distance are often correlated, geographic and genetic distances are often correlated ...). As I read your paper I thought "hmmm ... another colinearity problem", so perhaps, and just perhaps, it might be useful to use this framework of trying to distinguish which underlying variable is more important within a context of colinearity. One feature of colinearity is that explanatory power of a variable depends on the order in which it is entered into a sequential model that includes the other correlated variable or, in a simultaneous model, the presence or absence of the other variable. This is, more or less, what you show in Figure 4, so you are already addressing the issue. Perhaps the "colinearity framework" could contribute to the clarity requested by reviewer 2.
Please revise your manuscript taking into account the comments of all reviewers.
If you have any questions about my comments (I am not very good at annotating pdf's), just ask, and I will try to obtain the opinion of a statistically savy peer rapidly.
sincerely,
Jacqui Shykoff
Download recommender's annotations