

The evolution of cells within organisms can be an important determinant of disease. This is especially clear in the emergence of tumors and cancers from the underlying healthy tissue. In the healthy state, homeostasis is maintained through complex regulatory processes that ensure a relatively constant population size of cells, which is required for tissue function. Tumor cells escape this homeostasis, resulting in uncontrolled growth and consequent disease. Disease progression is driven by further evolutionary processes within the tumor, and so is the response of tumors to therapies. Therefore, evolutionary biology is an important component required for a better understanding of carcinogenesis and the treatment of cancers. In particular, evolutionary theory helps define the principles of mutant evolution and thus to obtain a clearer picture of the determinants of tumor emergence and therapy responses.
The study by Raatz and Traulsen [1] makes an important contribution in this respect. They use mathematical and computational models to investigate trait evolution in the context of evolutionary rescue, motivated by the dynamics of cancer, and also bacterial infections. This study views the establishment of tumors as cell dynamics in harsh environments, where the population is prone to extinction unless mutants emerge that increase evolutionary fitness, allowing them to expand (evolutionary rescue). The core processes of the model include growth, death, and mutations. Random mutations are assumed to give rise to cell lineages with different trait combinations, where the birth and death rates of cells can change. The resulting evolutionary trajectories are investigated in the models, and interesting new results were obtained. For example, the turnover of the population was identified as an important determinant of trait evolution. Turnover is defined as the balance between birth and death, with large rates corresponding to fast turnover and small rates to slow turnover. It was found that for fast cell turnover, a given adaptive step in the trait space results in a smaller increase in survival probability than for cell populations with slower turnover. In other words, evolutionary rescue is more difficult to achieve for fast compared to slow turnover populations. While more mutants can be produced for faster cell turnover rates, the analysis showed that this is not sufficient to overcome the barrier to the evolutionary rescue. This result implies that aggressive tumors with fast cell birth and death rates are less likely to persist and progress than tumors with lower turnover rates. This work emphasizes the importance of measuring the turnover rate in different tumors to advance our understanding of the determinants of tumor initiation and progression. The authors discuss that the well-documented heterogeneity in tumors likely also applies to cellular turnover. If a tumor consists of sub-populations with faster and slower turnover, it is possible that a slower turnover cell clone (e.g. characterized by a degree of dormancy) would enjoy a selective advantage. Another source of heterogeneity in turnover could be given by the hierarchical organization of tumors. Similar to the underlying healthy tissue, many tumors are thought to be maintained by a population of cancer stem cells, while the tumor bulk is made up of more differentiated cells. Tissue stem cells tend to be characterized by a lower turnover than progenitor or transit-amplifying cells. Depending on the assumptions about the self-renewal capacity of these different cell populations, the potential for evolutionary rescue could be different depending on the cell compartment in which the mutant emerges. This might be interesting to explore in the future.
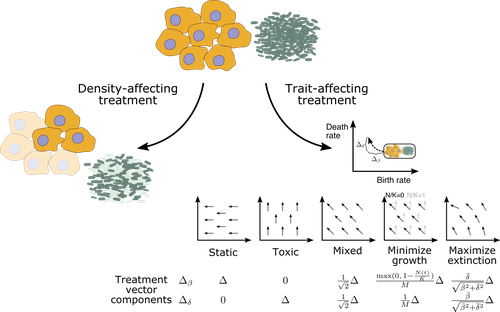
There are also implications for treatment. Two types of treatment were investigated: density-affecting treatments in which the density of cells is reduced without altering their trait parameters, and trait-affecting treatments in which the birth and/or death rates are altered. Both types of treatment were found to change the trajectories of trait adaptation, which has potentially important practical implications. Interestingly, it was found that competitive release during treatment can result in situations where after treatment cessation, the non-extinct populations recover to reach sizes that were higher than in the absence of treatment. This points towards the potential of adaptive therapy approaches, where sensitive cells are maintained to some extent to suppress resistant clones [2] competitively. In this context, it is interesting that the success of such approaches might also depend on the turnover of the tumor cell population, as shown by a recent mathematical modeling study [3]. In particular, it was found that adaptive therapy is less likely to work for slow compared to fast turnover tumors. Yet, the current study by Raatz and Traulsen [1] suggests that tumors are more likely to evolve in a slow turnover setting.
While there is strong relevance of this analysis for tumor evolution, the results generated in this study have more general relevance. Besides tumors, the paper discusses applications to bacterial disease dynamics in some detail, which is also interesting to compare and contrast to evolutionary processes in cancer. Overall, this study provides insights into the dynamics of evolutionary rescue that represent valuable additions to evolutionary theory.
References
[1] Raatz M, Traulsen A (2023) Promoting extinction or minimizing growth? The impact of treatment on trait trajectories in evolving populations. bioRxiv, 2022.06.17.496570, ver. 2 peer-reviewed and recommended by Peer Community in Evolutionary Biology. https://doi.org/10.1101/2022.06.17.496570
[2] Gatenby RA, Silva AS, Gillies RJ, Frieden BR (2009) Adaptive Therapy. Cancer Research, 69, 4894–4903. https://doi.org/10.1158/0008-5472.CAN-08-3658
[3] Strobl MAR, West J, Viossat Y, Damaghi M, Robertson-Tessi M, Brown JS, Gatenby RA, Maini PK, Anderson ARA (2021) Turnover Modulates the Need for a Cost of Resistance in Adaptive Therapy. Cancer Research, 81, 1135–1147. https://doi.org/10.1158/0008-5472.CAN-20-0806
DOI or URL of the preprint: https://doi.org/10.1101/2022.06.17.496570
Version of the preprint: 1
The paper seems very interesting, and in general is viewed positively by the reviewers. The reviewers make a number of useful suggestions and have some questions, which should be straightforward to address in a revision; this might benefit the paper.
This study investigates evolution during the early growth of tumours or bacterial populations and predicts which treatment strategies will most effectively steer the dynamics towards extinction. The analytical and numerical methods are well chosen. The paper is clearly written and logically structured. I have a few comments about the model assumptions. These concerns don’t necessarily require the generation of new results but I think they should at least be discussed in the paper.
Whereas the focus is on mutations that modify birth and death rates, and thus effective carrying capacities, isn’t it plausible also to have selection on the third trait, K? For example, bacteria could evolve to upregulate production of beneficial public goods, or cancer cells could evolve lower sensitivity to hypoxia, enabling them to achieve larger population sizes even while maintaining their initial basic birth and death rates. How might mutations modifying K change the results?
Mutation effects are assumed to be additive (equation 1), yet many other evolutionary models instead assume multiplicative effects. How might assuming multiplicative effects change the results? In the model “increasing both the initial birth and death rate equally, increases the number of extinct replicate population” (line 249) because “the same adaptation step in trait space gains a smaller increase in the survival probability of fast-turnover cells than in slow-turnover cells” (line 406). Would this result still hold if the model were to assume multiplicative effects?
How realistic and general is it to assume that “mortality during treatment is higher for less fit lineages” (line 462)? Chemotherapy, for example, targets the most rapidly dividing cells.
The choice of initial condition N(0) = 100 requires justification. In reality, every lineage begins with a single cell and much of the interesting dynamics occurs when the population size is below 100. If we assume the founder cell has equal birth and death rates, neglect density-dependent effects, ignore deleterious mutations (as the authors do throughout), and assume the population size follows a random walk (equivalent to gambler’s ruin) then the probability of this cell giving rise to a population of 100 cells before going extinct is 1%. That is, w/A, where w = 1 is the initial size and A = 100 is the target. So such lineages aren’t unreasonably rare. However, the shortest time until the population can grow from 1 to 100 cells is 99 generations and almost all lineages will take much longer than this. The expected waiting time is (w^2 – A^2)/3 = 3,333 generations. Given the assumed mutation rate of 0.005 per generation, almost all lineages will acquire multiple mutations by the time they reach 100 cells, and hence birth and death rates will vary both within and between 100-cell populations. Even if we instead assume that the birth rate is initially less than the death rate (inconsistent with homeostasis), it’s unclear whether 100-cell populations with equal birth and death rates will often arise. The authors should explain why they nevertheless chose to start with homogeneous 100-cell populations and discuss how this might limit the scope of their findings.
Minor comments:
Raatz and Traulsen use mathematical simulations to investigate the evolutionary dynamics of the cellular traits, birth and death rate, in the absence or presence of density- or trait-affecting treatment strategies. They find that adaptation follows a circular trajectory, increasing birth rates and lowering death rates, which favors lower turnover rates for evolutionary adaptation. If the creation of more mutant lineages also leads to higher evolvability is however determined by the treatment strategy determines. The authors further use geometric arguments to determine how different fitness components change the adaptive trajectories and suggest that net growth maximization could be a stronger determinant than survival probability.
Overall, this manuscript makes an important contribution to understanding the evolutionary landscape of crucial cellular traits, particularly under various treatment strategies. The authors present a thorough theoretical analysis and the manuscript is well-organized. As such I have only minor comments.
Strength:
A particular strength of the manuscript is its use of a geometric analysis that helps visualize the results and provides a nice way to extend the results without running every single simulation. The results of the analysis are additionally thoroughly tested using stochastic and deterministic simulations.
The authors study a range of different treatment strategies including bacteriostatic and bactericidal strategies, which is only considered in the minority of studies on evolution under treatment.
The results are generally presented in a clear and structured manner and connected to clinically relevant examples.
Weaknesses:
In some places the manuscript could benefit from a bit more clarification however:
- The abstract itself is to some extend difficult do understand as the reader doesn’t necessarily know what is meant by ‘circular adaptation trajectory’ and ‘geometrically derived hypotheses’.
- Where to the model parameter values come from and what exactly is the genetic variance in the birth and death rate intuitively? (Also, the table says genetic variance in death rate twice.)
- Similarly, is there empirical evidence for the truncated Gaussian distribution used for the mutated trait values.
- Where do the equations for minimizing growth and maximizing extinction in Figure 1 come from?
- The geometric presentation of treatment effects on different fitness effects could benefit from giving a bit more intuitive explanation on how the trait points and fitness isoclines change.
20000 seems to be quite low as a carrying capacity when thinking about bacterial populations and as the carrying capacity seems to have some influence on trait evolution, it would be good to discuss this parameter in realistic ranges as well.
Phenotypic plasticity is mentioned in the beginning of the results part but could be discussed a bit more compared to genetic changes.
The trait trajectories in Fig. 7b do not look extremely different. Maybe the authors could elaborate a bit more if they consider these differences still significant under clinical conditions.
In the discussion section (L435-438), the authors state that the observed prognosis regarding tumor growth does not fit with their observations. How do the authors explain this discrepancy?
https://doi.org/10.24072/pci.evolbiol.100555.rev12In this work the authors study a computational model for the evolution of cells under a different types of selective challenges. In their model cells can divide, die and mutate. Cell division is limited by a carrying capactiy, and mutations cause a random effect on birth and death rates. This model is developed to model the treatment of either cancer cells or bacteria. The authors consider a variety of treatment types. First there are what the author calls 'density-affecting' treatments, which immediately reduces the total population of cells. Second there are 'trait-affecting' treatments which can reduce cell birth rates (cyto-static), increase cell death rates (cyto-toxic), or affect both birth and death rates. The authors then study the evolution of the average birth death rates under a variety of treatment types. They find that 'density-affecting' treatments result in slower growing populations (conditional on survival) because of a less thorough exploration of trait-space. In contrast, 'trait-affecting' treatments will result in faster growing populations (conditional on survival). Overall, I thought this paper addressed interesting question in a reasonably clear fashion. Some of the critiques (some minor typos) I have are listed below.
1) Equation 1, summation should be over an index other than i.
2) Equation 3, I had a hard time understanding the role of 'f'. Perhaps they can add some more text clarifyting this function.
3) Page 7, lines 152-153. It seems like the process you described will never have negative cell numbers regardless of the effective carrying capacity. Cell deaths are proportional to the number of cells, so no cells means no deaths.
4) Page 7, line 157. 'not unambiguous' is a double negative.
5) Figure 4. The authors don't really provide an explanation of this phenomena until the discussion. I think it would be good to have some discussion of Figure 4 in Section 3.
6) Page 15, line 277. Label 'f' is re-used for a new purpose. Better to use a new symbol here.
7) Figure 7. I believe these are plots of population size conditioned on survival, however it's not clear. Please adjust the figure caption to state that these are plots conditioned on survival.
8) Page 23, lines 386-387. The authors should specify the evidence for circular adaptive trajectories in trait space, i.e., reference figures.
9) Page 24, line 410-415. I'm not convinced that the lower survival probability in high turnover cells is due to faster decline. In particular, mutations in your model are tied to births, and at least to me it would seem like the number of births prior to extinction should be the same regardless of turnover.
https://doi.org/10.24072/pci.evolbiol.100555.rev13
This paper aims to explore a general theoretical framework for understanding adaptation of population cells using a two-dimensional trait space consisting of birth and death rates. In the study, treatment that affects the population of cells is not explicitly modeled, but rather is characterized by its impacts on the population size vs birth and/or death rates. The work has interesting modeling ideas and biological realism could have been strengthened.
It would be helpful to validate the robustness of the closed-form approximation formula S1 with respect to its dependence on the carrying capacity K. K= 20 000 is used in the paper. If K gets too small, would that formula still hold?
Line 98 - 99: We assume that mutations in the two traits can occur independently and without correlation.
Would this be necessarily true? Should the cost of mutation (or some tradeoff between increasing birth rate and reducing death rate) be considered as well?
In the simulations, death rates approach to sufficiently small. If I understand correctly, would these living cells are now almost immortal and can live forever? This might be biologically implausible.
In the x-axis where death rate is zero, what is the direction of selection for birth rate? Can the birth rates (slowly or fast, depending on the model parameters) evolve as large as possible if one does not stop the simulation after t = 500.
To continue the comment above, what happens for much longer simulations? The current simulations stop at 500. If we let the simulation run as long as possible, what are the observations there?
Is there any potential conflict between minimizing extinction and maximizing growth? For simple one-stage birth-death models there seems no (as discussed in 3.3). But my intuition is that there might exist such a tradeoff in general multi-stage age-dependent reproduction models. See, e.g., though for a different system: https://onlinelibrary.wiley.com/doi/pdf/10.1111/ele.12392
Minor remarks
Line 20: Keywords include dormancy. But the present model does not address dormancy at all, if I understand correctly their model.
Line 65: These adaptations have lead to the development of drugs that
-> These adaptations have led to the development of drugs that
The paper draws insights from two big fields: cancer and bacteria (actually Schematic Figure 1 includes cancer cells and bacteria cells altogether). Although it is appealing to use general purpose models to shed light simultaneously on both systems, they are drastically different in their population dynamics. Thus it is very important to note this limitation when interpreting their findings in both fields, especially given that the present study is a conceptual study based entirely on simple birth-death models.
https://doi.org/10.24072/pci.evolbiol.100555.rev14