

The search for physiological markers of health and survival in wild animal populations is attracting a great deal of interest. At present, there is no (and may never be) consensus on such a single, robust marker but of all the proposed physiological markers, telomere length is undoubtedly the most widely studied in the field of evolutionary ecology (Monaghan et al., 2022).
Broadly speaking, telomeres are non-coding DNA sequences located at the end of chromosomes in eukaryotes, protecting genomic DNA against oxidative stress and various detrimental processes (e.g. DNA end-joining) and thus maintaining genome stability (Blackburn et al., 2015). However, in most somatic cells from the vast majority of the species, telomere sequences are not replicated and telomere length progressively declines with increased age (Remot et al., 2022). This shortening of telomere length upon a critical level is causally linked to cellular senescence and has been invoked as one of the primary causes of the aging process (López-Otín et al., 2023). Studies performed in both captive and wild populations of animals have further demonstrated that short telomeres (or telomere sequences with a fast attrition rate) are to some extent associated with an increased risk of mortality, even if the magnitude of this association largely differs between species and populations (Wilbourn et al., 2018).
The repeated observations of associations between telomere length and mortality risk have called for studies seeking to identify the ecological and biological factors that – beyond chronological age – shape the between-individual variability in telomere length. A wide spectrum of environmental stressors such as the level of exposure to pathogens or the degree of human disturbances has been proposed as possible modulators of telomere dynamics (see Chatelain et al., 2019). However, within species, the relative contribution of various ecological and biological factors on telomere length has been rarely quantified. In that context, the study of Criscuolo and colleagues (2023) constitutes a timely attempt to decipher the relative contribution of environmental and biological factors driving telomere length in nestlings (i.e. when individuals are between 15 and 35 days of age) from a wild population of little owls, Athene noctua.
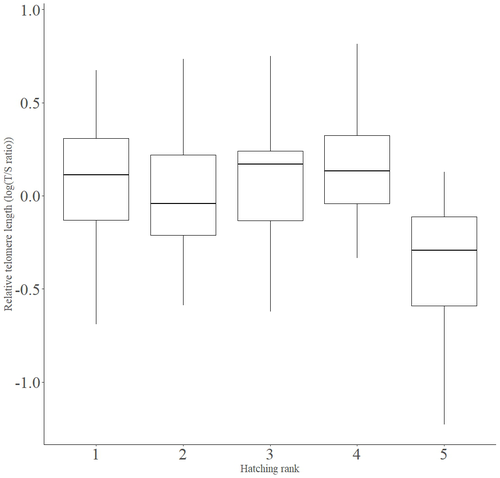
In addition to chronological age, Criscuolo and colleagues (2023) analysed the effects of two environmental variables (i.e. cohort and habitat quality) as well as three life history traits (i.e. hatching rank, sex and body condition). Among these traits, sex was found to impact nestling’s telomere length with females carrying longer telomeres than males. Traditionally, the among-individuals variability in telomere length during the juvenile period is interpreted as a direct consequence of differences in growth allocation. Fast-growing individuals are typically supposed to undergo more cell divisions and a higher exposure to oxidative stress, which ultimately shortens telomeres (Monaghan & Ozanne, 2018). Whether - despite a slightly female-biased sexual size dimorphism - male little owls display a condensed period of fast growth that could explain their shorter telomere is yet to be determined. Future studies should also explore the consequences of these sex differences in telomere length in terms of mortality risk. In birds, it has been observed that telomere length during early life can predict lifespan (see Heidinger et al., 2012 in zebra finches, Taeniopygia guttata), suggesting that females little owls might live longer than their conspecific males. Yet, adult mortality is generally female-biased in birds (Liker & Székely, 2005) and whether little owls constitute an exception to this rule - possibly mediated by sex-specific telomere dynamics - remains to be explored.
Quite surprisingly, the present study in little owls did not evidence any clear effect of environmental conditions on nestling’s telomere length, at both temporal and special scales. While a trend for a temporal effect was detected with telomere length being slightly shorter for nestling born the last year of the study (out of 4 years analysed), habitat quality (measured by the proportion of meadow and orchards in the nest environment) had absolutely no impact on nestling telomere length. Recently published studies in wild populations of vertebrates have highlighted the detrimental effects of harsh environmental conditions on telomere length (e.g. Dupoué et al., 2022 in common lizards, Zootoca vivipara), arguing for a key role of telomere dynamics in the emerging field of conservation physiology. While we can recognize the relevance of such an integrative approach, especially in the current context of climate change, the study by Criscuolo and colleagues (2023) reminds us that the relationships between environmental conditions and telomere dynamics are far from straightforward. Depending on the species and its life history, telomere length in early life could indeed capture very different environmental signals.
References
Blackburn, E. H., Epel, E. S., & Lin, J. (2015). Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science, 350(6265), 1193-1198. https://doi.org/10.1126/science.aab3389
Chatelain, M., Drobniak, S. M., & Szulkin, M. (2019). The association between stressors and telomeres in non-human vertebrates: A meta-analysis. Ecology Letters, 23, 381-398. https://doi.org/10.1111/ele.13426
Criscuolo, F., Fache, I., Scaar, B., Zahn, S. & Bleu, J. (2023). Telomere length vary with sex, hatching rank and year of birth in little owls, Athene noctua. EcoEvoRxiv, ver.4, peer-reviewed and recommended by PCI Evol Biol. https://doi.org/10.32942/X2BS3S
Dupoué, A., Blaimont, P., Angelier, F., Ribout, C., Rozen-Rechels, D., Richard, M., & Le Galliard, J. F. (2022). Lizards from warm and declining populations are born with extremely short telomeres. Proceedings of the National Academy of Sciences, 119(33), 2201371119. https://doi.org/10.1073/pnas.2201371119
Heidinger, B. J., Blount, J. D., Boner, W., Griffiths, K., Metcalfe, N. B., & Monaghan, P. (2012). Telomere length in early life predicts lifespan. Proceedings of the National Academy of Sciences, 109(5), 1743-1748. https://doi.org/10.1073/pnas.1113306109
Liker, A., & Székely, T. (2005). Mortality costs of sexual selection and parental care in natural populations of birds. Evolution, 59(4), 890-897. https://doi.org/10.1111/j.0014-3820.2005.tb01762.x
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2023). Hallmarks of aging: An expanding universe. Cell, 186(2), 243-278. https://doi.org/10.1016/j.cell.2022.11.001
Monaghan, P., Olsson, M., Richardson, D. S., Verhulst, S., & Rogers, S. M. (2022). Integrating telomere biology into the ecology and evolution of natural populations: Progress and prospects. Molecular Ecology, 31(23), 5909-5916. https://doi.org/10.1111/mec.16768
Monaghan, P., & Ozanne, S. E. (2018). Somatic growth and telomere dynamics in vertebrates: Relationships, mechanisms and consequences. Phil. Trans. R. Soc. B, 373(1741), 20160446. https://doi.org/10.1098/rstb.2016.0446
Remot, F., Ronget, V., Froy, H., Rey, B., Gaillard, J., Nussey, D. H., & Lemaitre, J. (2022). Decline in telomere length with increasing age across nonhuman vertebrates: A meta‐analysis. Molecular Ecology, 31(23), 5917-5932. https://doi.org/10.1111/mec.16145
Wilbourn, R. V., Moatt, J. P., Froy, H., Walling, C. A., Nussey, D. H., & Boonekamp, J. J. (2018). The relationship between telomere length and mortality risk in non-model vertebrate systems: A meta-analysis. Phil. Trans. R. Soc. B, 373(1741), 20160447. https://doi.org/10.1098/rstb.2016.0447
DOI or URL of the preprint: https://doi.org/10.32942/X2BS3S
Version of the preprint: 3
I have now read the revised version of the manuscript ‘Telomere length vary with sex, hatching rank and year of birth in little owls, Athena noctua’.
Authors have carefully addressed the comments raised by the two reviewers and I have no doubt that this manuscript will constitute a relevant contribution to the ‘evolutionary ecology of telomere dynamics’ literature.
I have a few minor comments on this revised version that authors should be able to address easily and rapidly.
- Line 45: Consider quoting Armstrong & Boonekamp 2018 (Ageing Research Reviews 85:101854)
- Line 52-54: Very complex sentence. Please rephrase / split in 2.
- Line 58: Could give one or two examples of ‘challenging conditions’
- Line 59-60: Could you be more specific here? What is ‘somatic maintenance’ in the context of telomere (e.g. telomerase expression, antioxidant mechanisms…)?
- Line 128: ‘only broods with more than 1 chick’ but you wrote in the previous sentence that all broods hade more hat 1 chick, so you basically included all broods....
- Line 166: ‘To identify traits shaping inter-individual variation in body condition…..’
- Lines 175-176: But it the Null model is less than 2 AIC points above the model with the lowest AIC, all parameters from the model with the lowest AIC will be non-significant (with alpha = 5%).
- Lines 170-176: Need to be a bit more specific here. What are the random effects? What are the categorical traits?
- Line 210: Missing space (also line 294).
- Figure 1 legend: For clarity purpose, please state in the legend that positive estimates correspond to longer telomere, negative estimates to shorter telomere.
- Result section: Please add a table with all the effect size and 95% CI.
- Figures 2 and 3: Could you please specify whether these figures control for all the other factors included in the model (i.e. factors displayed in figure 1). Could you also provide the sample size for each group on both figures 2 and 3.
- Lines 299-302: But is there an association between body mass and nestling telomere length here?
- Lines 311-317: Could you rephrase here, I can’t see a case where parents should consistently favor one sex over the other? This should ultimately lead to some forms of sexual conflict no?
- Lines 331-33: It is more than a non-significant effect, there is a tendency for shorter telomeres in meadow and orchards environment. I suggest to add a few sentences regarding the absence of environmental quality early in life (e.g. do meadows and orchards really represent such high-quality environments?).
I hope these comments will be helpful.
Kind regards,
JF Lemaître
DOI or URL of the preprint: https://doi.org/10.32942/X2BS3S
Version of the preprint: 2
Dear François and co-authors
I have now received reviews from two referees. Based on these two reports and my own reading of the paper I am willing to recommend your paper. However, reviewers have raised important points that need to be addressed in a revised version. Taking these comments into account will definitely clarify the magnitude and the robustness of the relationships documented in your paper. This needs to be done before I can recommend the preprint.
I also have a few additional remarks. Some of these comments echo those of the referees
- Lines 90-92: Agree but this requires to test an interaction between laying order and environmental conditions which is not what has been done in your analyses.
- Lines 96- 96: Please rephrase as environmental conditions is unlikely to influence the sex of the individuals. More generally, I don’t understand the rational of this analysis as the whole introduction is focused on the factors modulating telomere length. This comes rather out of the blue.
- Lines 122-124: Please give the units
- Line 127: I assume this contains broods with 1 chick, you should rephrase the sentence as: ‘The original dataset contained 142 nestlings….”
- Line 167: Please give the formula of the ScaleMassIndex
- Lines 171-172: What is the justification for performing a model averaging approach.
- Lines 190-193: This is due to the fact that the constant model has the lowest AIC. This model should be retained. I don’t see the justification for performing model averaging in such a situation.
- Lines 283-284: They likely face the trade-off but differences in individual quality might explain the pattern.
I hope that all these comments will be helpful,
Kind regards,
Jean-François Lemaître
Review: Telomere length vary with sex, hatching order and year of birth in little owls, Athene noctua
Comment to the Authors:
This paper presents an analysis of the effect of hatching rank, body condition, sex and nest environment on telomere length using 4 years of data on a raptor species, Athene noctua. The results suggest that telomeres are longer in females and in individual in better body condition. In addition, authors found, following their prediction, a negative effect of hatching rank on telomere length. This effect was due to shorter telomere in the last-hatched individuals in large clutches. Finally, while no effect of the environment was found on telomere length, telomere length seems to decrease over the 4 years of sampling. The manuscript is nice to read and presents interesting results, however, I feel like the result section lacks basic information (as described in my detailed comments to the authors). Relationships seem to be weak and authors should be more cautious about their findings. The result section should be more detailed and more complete (e.g. adding a table with estimates, SE and confidence interval for explanatory variable). My detailed comments are presented below, and I hope they will be helpful to the authors in revising their manuscript.
Florentin Remot (please note that I sign all my reviews)
My main concerns are:
Minor comments:
Lines 127-129: Sample size are quite small for each level (especially for broods with 2 and 5 chicks), In my opinion, it is hard to tell whether the absence of effect of nestling number is biologically meaningful or is due to low power. In addition, the effect of nestling number could be tested separately by also using clutches containing 1 chick.
Line 172: Could you be more precise regarding the model averaging procedure? Which package/function did you use? Your estimates were from conditional or full averaging?
Line 210: Is the positive effect of SMI on RTL statistically significant? It is not clear on the figure. As I previously said in the general comment above, you present as supplementary tables the list of the best models selected based on AICc, however you then use a model averaging procedure for your results. Thus, it’ll be more informative to add in the main manuscript the table with the estimate and the 95% CI calculated from the model averaging.
Figure 2: The sex difference is not clear on the figure; my suggestion would be to remove the figure or to represent the sex difference differently (e.g., with a pairwise contrast plot).
Figure 3: The cohort effect is not clear on the plot. I would suggest plotting the cohort effect and the hatching order effect separately.
Line 233: The use of the word ‘longitudinal’ here is a bit confusing as it is not a longitudinal follow-up. I would suggest dropping this term from the sentence since it could be misleading for the reader.
I would suggest authors to be consistent with variable names, for instance ‘hatching order’ in Figure 1 but ‘Rank’ in table S3, as it might be confusing for the reader.