

 based on reviews by Danna Gifford, Helen Alexander and 1 anonymous reviewer
based on reviews by Danna Gifford, Helen Alexander and 1 anonymous reviewer
The increase in the prevalence of (antibiotic) resistance has become a major global health concern and is an excellent example of the impact of real-time evolution on human society. This has led to a boom of studies that investigate the mechanisms and factors involved in the evolution of resistance, and to the spread of the concept of "costs of resistance". This concept refers to the relative fitness disadvantage of a drug-resistant genotype compared to a non-resistant reference genotype in the ancestral (untreated) environment.
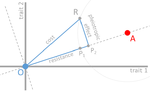
In their paper, Lenormand et al. [1] discuss the history of this concept and highlight its caveats and limitations. The authors address both practical and theoretical problems that arise from the simplistic view of "costly resistance" and argue that they can be prejudicial for antibiotic resistance studies. For a better understanding, they visualize their points of critique by means of Fisher's Geometric model.
The authors give an interesting historical overview of how the concept arose and speculate that it emerged (during the 1980s) in an attempt by ecologists to spread awareness that fitness can be environment-dependent, and because of the concept's parallels to trade-offs in life-history evolution. They then identify several problems that arise from the concept, which, besides the conceptual misunderstandings that they can cause, are important to keep in mind when designing experimental studies.
The authors highlight and explain the following points:
1. Costs of resistance do not necessarily imply pleiotropic effects of a resistance mutation, and pleiotropy is not necessarily the cause of fitness trade-offs.
2. Any non-treated environment and any treatment dose can result in a different cost.
3. Different reference genotypes may result in different costs. Specifically, the reference genotype has to be "optimally" adapted to the reference environment to provide an accurate measurement of costs.
Lenormand et al.'s paper [1] is a timely perspective piece in light of the ever-increasing efforts to understand and tackle resistance evolution [2]. Although some readers may shy away from the rather theoretical presentation of the different points of concern, it will be useful for both theoretical and empirical readers by illustrating the misconceptions that can arise from the concept of the cost of resistance. Ultimately, the main lesson to be learned from this paper may not be to ban the term "cost of resistance" from one's vocabulary, but rather to realize that the successful fight against drug resistance requires more differential information than the measurement of fitness effects in a drug-treated vs. non-treated environment in the lab [3-4]. Specifically, a better integration of the ecological aspects of drug resistance evolution and maintenance is needed [5], and we are far from a general understanding of how environmental factors interact and influence an organism's (absolute and relative) fitness and the effect of resistance mutations.
References
[1] Lenormand T, Harmand N, Gallet R. 2018. Cost of resistance: an unreasonably expensive concept. bioRxiv 276675, ver. 3 peer-reviewed by Peer Community In Evolutionary Biology. doi: 10.1101/276675
[2] Andersson DI and Hughes D. Persistence of antibiotic resistance in bacterial populations. 2011. FEMS Microbiology Reviews, 35: 901-911. doi: 10.1111/j.1574-6976.2011.00289.x
[3] Chevereau G, Dravecká M, Batur T, Guvenek A, Ayhan DH, Toprak E, Bollenbach T. 2015. Quantifying the determinants of evolutionary dynamics leading to drug resistance. PLoS biology 13, e1002299. doi: 10.1371/journal.pbio.1002299
[4] Bengtsson-Palme J, Kristiansson E, Larsson DGJ. 2018. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiology Reviews 42: 68–80. doi: 10.1093/femsre/fux053
[5] Hiltunen T, Virta M, Laine AL. 2017. Antibiotic resistance in the wild: an eco-evolutionary perspective. Philosophical Transactions of the Royal Society B: Biological Sciences 372: 20160039. doi: 10.1098/rstb.2016.0039
DOI or URL of the preprint: https://doi.org/10.1101/276675
Version of the preprint: 1
 , posted 17 May 2018
, posted 17 May 2018This Perspective was reviewed by 3 external reviewers, with whom l agree that a criticism of the misleading use ot the term "costs of resistance" is a timely issue and that this manuscript can be of relevance for both empirical and theoretical studies. However, all reviewers provided excellent suggestions that would allow for the manuscript to reach a larger target audience and that would improve its clarity. Most importantly, all reviewers suggest that the link to empirical studies and the implication for such studies needs to be more developed, and that there should be more concrete suggestions on on how to move beyond the term cost both theoretically and empirically. The reviewers also provide several interesting references that may complement the literature review provided by the authors.
Although we appreciate Reviewer 3's concern that complementing the existing discussion via Fisher's Geometric model (FGM; which should indeed be defined to the "naive" reader) by a discussion of models based on dose-response curves may be illustrative and helpful for readers less familiar with FGM, we feel that this may go beyond the scope of the current manuscript. However, this alternative and commonly considered model of fitness effects across environments should be discussed.
For people unfamiliar with FGM, it could also be helpful to indicate the important aspects of the model in each figure directly, i.e., "Optimum AB-" instead of "O", etc.
In this Perspective, the authors tackle a juggernaut of the applied evolution literature, the “cost of antibiotic resistance”. Their essential argument is that describing resistance mutations as “costly” in the absence of antibiotics is an oversimplification that has lead the field astray—essentially, we ought to be considering fitness effects of resistance mutations in different environments, as we would for any other class of mutation.
I think this perspective is worthwhile, though perhaps a touch adversarial. The field at large does appear to be aware of the fact that resistance is not always costly (but they are “generally costly”). I suppose I would like the authors to address why resistance isn’t the default state for wild-type organisms, if resistance isn’t generally costly.
I think the section on considering the fitness effects of resistance in non-optimal genetic backgrounds is the most interesting and important aspect of this work. There are certainly specific situations where resistance mutations provide a benefit in non-optimal organisms (e.g. Kassen and Bataillon 2006, which the authors cite, although Bataillon et al. 2011 showed that many of the strains are not single mutants; and some examples in the rifampicin resistance literature e.g. Rodriguez-Verdugo et al. BMC Evol Biol doi:10.1186/1471-2148-13-50). The authors do an excellent job outlining this conceptually from FGM, but I do wonder about the strength of support from data---though one could certainly argue that the data are biased toward finding costs, due to the popularity of the concept of “costs of resistance” itself.
(A minor point, but the manuscript would benefit from minor copy editing to fix consistency in usage of e.g. single vs. double quotes, subject/verb agreement, grammar, etc.)
Section “Resistance mutations as beneficial mutations” The statement “In brief, resistance mutation are beneficial mutations.” is oversimplified and should be qualified with “in the presence of antibiotic”, otherwise it would seem to suffer from the point the authors are trying to make, that context is everything.
Section “The context dependence of fitness effects” “This selective advantage is not easy to estimate in the field, but is often thought to represent an inherent property of the mutation itself.” Nevertheless, there is parallelism in terms of the specific resistance alleles that are observed in clinical isolates, particularly notable in the TB rifamycin resistance literature, where recombination is thought to be low.
Section “Costs of resistance are not pleiotropic effects” After reading this section, I did not fully grasp the argument that costs of resistance are not due to pleiotropy. Certainly some resistance mutations are highly pleiotropic (e.g. rpoB resistance, which globally affects gene expression levels and patterns, Qi et al. 2014, doi:10.1128/mBio.01562-14).
Figure 1: please identify P1 and P2 in the legend text.
Section “Resistance mutations do not have a cost” Might I suggest modifiying this to “Resistance mutations do not always have a cost”? Clearly sometimes resistance mutations do/can have a cost relative to the wild-type, as demonstrated in Figures 1 and 2. I think a point worth addressing would be, if resistance mutations do not generally have a cost, why is resistance not the default state for organisms?
At the risk of appearing self-serving, the authors may find interest our work on the effects of rifampicin resistance mutations at a single locus under different environmental conditions (temperature, carbon source, Gifford et al. 2016, doi:10.1111/evo.12880), which I think demonstrates the authors’ essential point: resistance mutations are not necessarily deleterious, and that compensated resistance is not always fully restorative. The authors may also like a recent publication looking at genetically diverse E. coli from Basra et al. (2018, GBE, doi:10.1093/gbe/evy030).
Figure 2: Please identify P1 and P3(?) in the figure legend.
Section “What is a resistance mutation?” “If resistance mutations cannot be defined by the fact that they are beneficial in the treated environment”: it would be helpful to have a citation for this definition for resistance. I believe that a more commonly used definition is a mutation (or gene) that allows growth at a specific concentration of antibiotic, in the genetic background otherwise incapable of growing at that concentration.
The perspective by Lenormand and co-authors provides an interesting short historical overview of the term “cost of resistance”, the theoretical limitations of the term and discuss the academic usefulness of it. The authors present valid and clear arguments in a well-written manuscript and I enjoyed reading it. Moreover, I agree with the authors that the term “cost of resistance” has serious limitations. For instance, resistance mutations are indeed usually considered pleotropic (in traits) and the direct cost of the mutations is often forgotten. The cost of resistance in fact, is not the same entity as the “cost” of pleiotropy.
However, I feel that the perspective is somewhat biased. For instance, abolishing the term will not solve of the issues that fitness effects are dependent on the environment. Which environments are more relevant to study remains an open question since it is not possible to study fitness effects directly in some of the relevant environments (i.e.: the human host). Even though lots of problems arise when fitness costs are measured in vitro (i.e. in the test tube) and it is difficult to choose the right environment to study, it has led to the useful predictions that resistance mutations with lower costs should be more prevalent in clinical isolates of some pathogens. This was indeed observed for clinical isolates of Mycobacterium tuberculosis (Gagneux et al., Science 2006).
Moreover, the authors have not discussed the practical applications of the term “cost of resistance”. For instance, this term has been helpful raising awareness to the problem of antibiotic resistance and it has influenced political decisions such as to halt the use of certain antimicrobials with observed decrease of antimicrobial resistance in clinical settings (Seppälä et al., 1997, N. Engl. J. Med. 337, 441–446; Enne et al., 2001 Lancet 357, 1325–1328; Bean et al., 2005 J. Antimicrob. Chemother. 56, 962–964; Gottesman et al., 2009 Clin. Infect. Dis. 49, 869–875).