
Facultative parthenogenesis and transitions from sexual to asexual reproduction
 based on reviews by 3 anonymous reviewers
based on reviews by 3 anonymous reviewers
Facultative parthenogenesis: a transient state in transitions between sex and obligate asexuality in stick insects?
Abstract
Recommendation: posted 10 April 2023, validated 11 April 2023
Bilde, T. (2023) Facultative parthenogenesis and transitions from sexual to asexual reproduction . Peer Community in Evolutionary Biology, 100551. 10.24072/pci.evolbiol.100551
Recommendation
Despite a vast array of ways in which organisms can reproduce (Bell, 1982), most animals engage in sexual reproduction (Otto & Lenormand, 2002). A fascinating alternative to sex is parthenogenesis, where offspring are produced asexually from a gamete, typically the egg, without receiving genetic material from another gamete (Simon, Delmotte, Rispe, & Crease, 2003). One of the long-standing questions in the field is why parthenogenesis is not more widespread, given the costs associated with sex (Otto & Lenormand, 2002). Natural populations of most species appear to be reproducing either sexually or parthenogenetically, even if a species can employ both reproductive modes (Larose et al 2023). Larose et al (2023) highlight the conundrum in this pattern, as organisms that are capable of employing parthenogenesis facultatively would be able to gain the benefits of both modes of reproduction. Why then, is facultative parthenogenesis not more common?
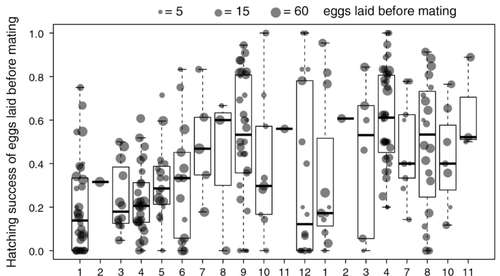
Larose et al (2023) propose that constraints on being efficient in both sexual and asexual reproduction could cause a trade-off between reproductive modes that favours an obligate strategy of either sex or no sex. This would provide an explanation for why facultative parthenogenesis is rare. Timema stick insects provide an excellent system to investigate reproductive strategies, as some species have parthenogenetic females, while other species are sexual, and they show repeated transitions from sexual reproduction to obligate parthenogenesis (Schwander & Crespi, 2009). The authors performed comprehensive and complementary studies in a recently discovered species T. douglasi, in which populations show both modes of reproduction, with some populations consisting only of females and others showing equal proportions of males and females. The sex ratio varied significantly, with the proportion of females ranging between 43-100% across 29 populations. These populations form a monophyletic clade with clustering into three genetic lineages and only a few cases of admixture. Females from all populations were capable of producing unfertilized eggs, but the hatching success varied hugely among populations and lineages (3-100%). Parthenogenetically produced offspring were homozygous, showing that parthenogenesis causes a complete loss of heterozygosity in a single generation. After producing eggs as virgins, females were mated to assess the capacity to also reproduce sexually, and fertilization increased the hatching success of eggs in two lineages. In one lineage, in which the hatching success of unfertilized eggs is similar to that of other sexually reproducing Timema species, fertilization reduced egg-hatching success, indicating a trade-off between reproductive modes with parthenogenetic reproduction performing best. Approximately 58% of the offspring produced after mating were fertilized, demonstrating the capacity of females to reproduce parthenogenetically also after mating has occurred, however with huge variation among individuals.
This wonderful and meticulously performed study produces strong and complementary evidence for facultative parthenogenesis in T. douglasi populations. The study shows large variation in how reproductive mode is employed, supporting the existence of a trade-off between sexual and parthenogenetic reproduction. This might be an example of an ongoing transition from sexual to asexual reproduction, which indicates that obligate parthenogenesis may derive via transient facultative parthenogenesis.
REFERENCES
Bell, G. (1982) The Masterpiece of Nature: The Evolution and Genetics of Sexuality. University of California Press. 635 p.
Otto, S. P., & Lenormand, T. (2002). Resolving the paradox of sex and recombination. Nature Reviews Genetics, 3(4), 252-261. https://doi.org/10.1038/nrg761
Schwander, T., & Crespi, B. J. (2009). Multiple direct transitions from sexual reproduction to apomictic parthenogenesis in Timema stick insects. Evolution, 63(1), 84-103.
https://doi.org/10.1111/j.1558-5646.2008.00524.x
Simon, J.-C., Delmotte, F., Rispe, C., & Crease, T. (2003). Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals. Biological Journal of the Linnean Society, 79(1), 151-163. https://doi.org/10.1046/j.1095-8312.2003.00175.x
Larose, C., Lavanchy, G., Freitas, S., Parker, D.J., Schwander, T. (2023) Facultative parthenogenesis: a transient state in transitions between sex and obligate asexuality in stick insects? bioRxiv, 2022.03.25.485836, ver. 4 peer-reviewed and recommended by Peer Community in Evolutionary Biology. https://doi.org/10.1101/2022.03.25.485836
The recommender in charge of the evaluation of the article and the reviewers declared that they have no conflict of interest (as defined in the code of conduct of PCI) with the authors or with the content of the article. The authors declared that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
We would like to acknowledge funding from the European Research Council (Consolidator Grant No Sex No Conflict), Swiss FNS grant 31003A_182495, and the University of Lausanne
Evaluation round #2
DOI or URL of the preprint: https://doi.org/10.1101/2022.03.25.485836
Version of the preprint: 3
Author's Reply, 29 Mar 2023
Dear Recommender
Thanks a lot for your comments. We have changed the sentence "Obligately parthenogenetic Timema have also lost all heterozygosity, suggesting that parthenogenesis evolved via a gradual capacity increase while conserving the proximate mechanism." to "Obligately parthenogenetic Timema have also lost all heterozygosity, suggesting that the transition to obligate parthenogenesis did not require a modification of the proximate mechanism, but rather involved a gradual increase in frequency."
Regarding the second part of the comment (explaining the mechanism): We think that it is safer not to point to a specific proximate mechanism. We carefully avoided doing so in the manuscript as the heterozygosity patterns we observe could in theory be explained by different mechanisms (namely gamete duplication, terminal fusion with no recombination (or only at the telomeres), or central fusion with recombination at the centromeres).
I hope this adresses your comment.
Best regards,
Guillaume Lavanchy
Decision by Trine Bilde , posted 23 Mar 2023, validated 23 Mar 2023
, posted 23 Mar 2023, validated 23 Mar 2023
Dear Guillaume and Tanja
Thank you for the thorough revision of your manuscript. I am very pleased to recommend the pre-print.
I had only one comment to the following sentence in the abstract (the point is also made in the discussion).
"Obligately parthenogenetic Timema have also lost all heterozygosity, suggesting that parthenogenesis evolved via a gradual capacity increase while conserving the proximate mechanism."
It is not so easy to follow this sentence, would it be possible to explain in more detail what is meant by 'gradual capacity incrase' and also explain the proximate mechanism?
Thank you,
Best regards
Trine
Evaluation round #1
DOI or URL of the preprint: https://doi.org/10.1101/2022.03.25.485836
Version of the preprint: 2
Author's Reply, 15 Mar 2023
Decision by Trine Bilde , posted 21 Jun 2022
, posted 21 Jun 2022
Dear Authors
I have read this interesting manuscript, and have also obtained reviews from three experts.
The reviewers and I are excited about your paper, and they have provided very constructive comments aimed to improve clarity and presentation.
I invite you to revise the manuscript by addressing the comments provided.
Reviewed by anonymous reviewer 1, 16 Jun 2022
This preprint assesses variation in reproductive modes in a species of stick insects, so far believed to reproduce by obligate asexuals. The authors demonstrate through multiple lines of evidence that the two investigated locations are in fact inhabited by three lineages of facultative asexuals with one lineage (one location) being much closer to obligate asexuality than the other two lineages (both from the other location). The findings further show that asexuality occurs by mode that leads to complete or almost complete autozygosity in one generation (gamete fusion or terminal fusion without recombination or similar mechanisms; see a new paper by Archetti for the possibility of inverted meiosis, where complete homozygosity may also occur through suppression of meiosis I in absence of recombination). In addition, there is some evidence for rare interbreeding with a related sexual species (mostly from discordance of nuclear and mitochondrial phylogenies), as well as for a potential trade-off between sexual and asexual reproduction (individuals of the more “obligate” parthenogenetic lineage have poor hatching success after fertilization). Taken together, this is a detailed investigation into facultative parthenogenesis in an insect species, whose relatives are known to show a variety of reproductive modes including “obligate sexuality” with rare asexual reproduction and obligate asexuality (even though in the light of this study one wonders whether evidence for “obligate” reproductive modes shouldn’t be questioned also in other species). The results are discussed in light of the hypothesis that facultative parthenogenesis might be an intermediate state between obligate sex and obligate asex (but the results themselves cannot resolve this question neither whether these facultative asexual evolved from sexual or asexual ancestors.
Overall, the manuscript is very well written and clear, and the experimental, genetic, and statistical approaches are sound. I have almost never seen a manuscript on which I had as few specific, minor comments for possible improvements as for this one. However, there are two issues that diminish, in my opinion, the suitability for PCI recommendation in the current version (I feel that both these points would be relatively straightforward to address in a revision). First, parts of the manuscript, especially large parts of introduction and discussion are too system-specific, and it may be good to present the manuscript from the beginning from a somewhat larger, more general perspective to increase the overall scope of the study. Second, and related to the previous point, parts of the discussion are not very well referenced and comparisons with other study systems could be strengthened. Neiter of the points, however, requires extensive changes.
Specific points:
The sentence on L. 69-71 is cryptic (unless one has already read the study)
L. 162: How was this DNA obtained? From collaborators? From authors of a previous study?
L. 230: hemizygous rather than homozygous
L. 457: Or perhaps just variation in the rate of recombination (not two distinct parthenogenesis mechanisms).
I may have missed it, but I don’t remember that the previous evidence for “obligate” parthenogenesis in the species is discussed in any detail nor where (geographically) these putatively obligate populations occur.
Reviewed by anonymous reviewer 2, 16 Jun 2022
This paper presents new results regarding the reproductive modes of the North American stick insect Timema douglasi. It is based on field sampling on two transects, experiments to investigate reproductive modes and extensive genomic data. The key result is that this taxon, originally thought to be exclusively parthenogenetic, is in fact able to reproduce sexually, although to a different extent depending on the genotype.
I read this manuscript with interest. This is yet another case study showing that asexuals are not reproducing in the way initially envisioned. The demonstration is convincing and the data supports the main finding without ambiguity.
I have however some reservations regarding the interpretation and some of the analyses. I think they can be addressed in a revision.
Main comments
1- Overall, the authors present and discuss their work by asking whether it represents a case of vestigial sex or ‘re-evolution’ of sex, even invoking “Dollo’s law of irreversibility” for the latter. I think that this is casting the problem in an unnecessarily complicated way. I would suggest to cut the part on Dollo’s law. This “law” is of very little interest as it is based on no clear process. It is just a mere label for disparate observations without real content. More fundamentally, the results might be interpreting differently, by simply saying that these timemas are all facultative parthenogens, although to a different extent across genotypes. I think it is possible to make this case much more strongly. In fact, there is some inconsistency in the reasoning that pre-date this paper. The term “tycho-parthenogenesis” has been introduced to depict situations where sexual females, could lay viable unfertilized eggs. This occurs for instance in absence of mates. The observation that these females are capable of reproducing by parthenogenesis and sexually is basically the same as the observations made in this paper. The only difference is quantitative, not qualitative. Often, the term tychoparthenogenesis is used for low rates of parthenogenesis, but it must involve a mechanism of producing diploid unfertilized eggs. So, apart from the rate of sex vs asex, is there really a difference between the mixed reproductive mode observed in the paper and the already well-known fact that these timema are capable of both sex and “tychoparthenogenesis”. It is very likely that the underlying mechanism are the same and that only sex-asex rates differ across different genotypes. Hence, one could make the relatively strong argument that the use of the label ‘tychoparthenogenesis’ has been misleading from the start, and that the current paper is simply documenting the fact that the rate of sex-asex varies more importantly than was previously considered. In this view, this is not a question of vestigial sex or ‘re-evolved’ sex. All T. douglasi have a mixed reproductive mode. It varies quantitatively across genotypes, but sex (and asex) were never ever really either lost of regained. The capacity for sex and asex may simply have been there, all along, in all lineages.
2- The paper does not clearly explain why the sex ratio varies strongly in Manchester transect between populations 7 and 8. Regarding the sampling and genotyping results, there are very few individual tested in populations 8-12 in Manchester transect (and none in population 8 and 9 where the sex ratio abruptly varies), which makes it hard to well understand what is going on. However, the transition in sex ratio is not mirrored by a genotypic transition, as one would expect. The main geographical pattern is therefore left unexplained in the paper, and this is certainly a missed opportunity. I think that it is possible to make the case that the yellow clade can be split between two subgroups with distinct reproductive modes. I have tried to look at this based on the Fig 1E and Fig 3, and it does seem to exist such a difference, which may really help to understand why the sex-ration varies so abruptly after population 7 : the yellow individuals seem to belong to different subgroups before and after this transition. I think it is necessary to better analyze what is going on. I understand that automatically separating clusters yields to these red/yellow/blue subgroups. However, I find it very surprising that the low heterozygosity individuals tend to cluster together in the phylogeny, suggesting that different yellow subgroups ay actually reproduce differently, perhaps explaining the geographic transition in Manchester transect. Even if this suggestion is not correct (it is only based on my limited ability to investigate this using the figure), it is necessary to better analyze and discuss it. In the end, the reader expect to see an explanation for the extraordinary sex-ratio pattern seen on Manchester transect. This should be a primary concern of the paper.
3- Regarding the results of the experiments illustrated on Fig 5, it would be important to clarify whether the hatching success varies between fertilized and non-fertilized eggs, especially for the yellow group. This information is not directly available, but a correspondence might be doable across females. This has to be clarified. Maybe splitting the yellow group in two subgroups as suggested above will also clarify the pattern shown on Fig. 5. Similarly for the blue group, there are individuals with or without heterozygosity and it would be important to know if they represent different subgroups with different reproductive modes. These considerations are not sufficiently detailed and thoroughly investigated.
4- In the discussion, the authors insist on the idea that sex and parthenogenesis are traded-off against each other (line 518). Can they produce a figure illustrating this directly? I do not see very well how the results can be used to directly support this claim. An important clarification is needed here.
Other, more specific comments
l184-188. Explain better the reason behind this hypothesis involving paralogues and missing data.
l245. Was it possible to remove PCR duplicates without paired-end sequencing information?
l261-264. Looking whether a value falls in an interval is not a proper test. Many tests are also performed here.
l501-504. This is, I think, an important observation indeed, supporting the view that these “lineages” may not be really lineages given that sex is relatively frequent. Hence a phylogenetic representation is probably misleading. Some discussion about this would be useful.









