
PANTE Eric
- Laboratory of Environmental Marine Sciences (LEMAR), CNRS, Plouzané, France
- Evolutionary Ecology, Phylogenetics / Phylogenomics, Phylogeography & Biogeography, Systematics / Taxonomy
- recommender
Recommendations: 2
Reviews: 0
Recommendations: 2

Cryptic species and hybridisation in corals: challenges and opportunities for conservation and restoration
How common cryptic coral diversity can blur biodiversity metrics and challenge management
Recommended by Eric Pante based on reviews by 2 anonymous reviewersBiological conservation aims at protecting the genetic diversity generated by evolutionary processes over the course of life's history on earth (Allendorf and Luikart 2007), and to be effective, it requires that its fundamental units (among which populations and species) be delimited as precisely as possible. This exercise is particularly important for corals because hybridisation and introgression have played a fundamental role in shaping their contemporary diversity (eg Veron 1995).
In their review paper, Riginos et al (2024) show that 68% of nominal taxa investigated for genomic population structure bear the molecular signature of partial reproductive isolation, and can be considered as cryptic genetic groups. Another review study (published a day before the preprint of Riginos et al), converges in the finding that cryptic diversity is rampant in nominal coral species (Grupstra et al 2024). This result has strong bearing on the study of coral biology; as Riginos et al state, "any coral investigation that does not genotype the corals under study risks treating a heterogeneous mix of partially reproductively isolated taxa as a single species." The stakes are therefore high, given the ecological importance of corals and the ecosystem services they provide.
While Grupstra et al (2024) discusses the impact of cryptic coral diversity in the context of functional differences in thermal adaptation and the processes that lead to cryptic lineages, Riginos et al (2024) provide a quantitative review of cryptic lineages within nominal species, providing reproducible criteria for delineation, details the importance of detecting hybrids, summarises how biodiversity metrics and conservation efforts can be biased by unrecognised cryptic lineages, offer recommendations on how to recognise and deal with cryptic diversity, and discuss how corals can be regarded as highly valuable model systems to study adaptation and speciation. The study of Riginos et al (2024) is therefore a must-read to all coral biologists, especially those involved in biological conservation.
References
Fred W. Allendorf and Gordon Luikart (2007) Conservation and Genetics of Populations. Blackwell Publishing, Malden MA, USA.
Carsten G. B. Grupstra, Matías Gómez-Corrales, James E. Fifer, Hannah E. Aichelman, Kirstin S. Meyer-Kaiser, Carlos Prada, Sarah W. Davies (2024) Integrating cryptic diversity into coral evolution, symbiosis and conservation. Nat Ecol Evol 8, 622–636 (2024). https://doi.org/10.1038/s41559-023-02319-y
Cynthia Riginos, Iva Popovic, Zoe Meziere, Vhon Garcia, Ilha Byrne, Samantha Howitt, Hisatake Ishida, Kevin Bairos-Novak, Adriana Humanes, Hugo Scharfenstein, Thomas Richards, Ethan Briggs, Vanessa Clark, Chuan Lei, Mariam Khan, Katharine Prata (2024) Cryptic species and hybridisation in corals: challenges and opportunities for conservation and restoration. EcoEvoRxiv, ver.2 peer-reviewed and recommended by PCI Evol Biol https://doi.org/10.32942/X2502X
J. E. N. Veron (1995) Corals in Space and Time: The Biogeography and Evolution of the Scleractinia. Cornell University Press
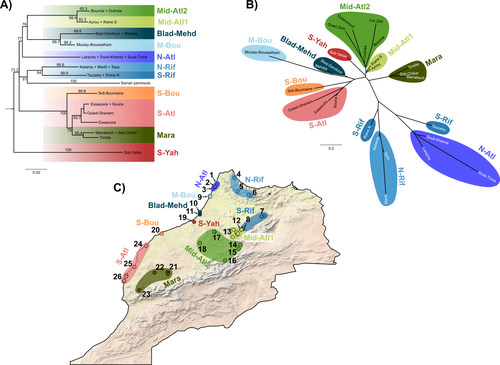
Phylogeographic breaks and how to find them: Separating vicariance from isolation by distance in a lizard with restricted dispersal
The difficult task of partitioning the effects of vicariance and isolation by distance in poor dispersers
Recommended by Eric Pante based on reviews by Kevin Sánchez and Aglaia (Cilia) AntoniouPartitioning the effects of vicariance and low dispersal has been a long-standing problem in historical biogeography and phylogeography. While the term “vicariance” refers to divergence in allopatry, caused by some physical (geological, geographical) or climatic barriers (e.g. Rosen 1978), isolation by distance refers to the genetic differentiation of remote populations due to the physical distance separating them, when the latter surpasses the scale of dispersal (Wright 1938, 1940, 1943).
Vicariance and dispersal have long been considered as separate forces leading to separate scenarii of speciation (e.g. reviewed in Hickerson and Meyer 2008). Nevertheless, these two processes are strongly linked, as, for example, vicariance theory relies on the assumption that ancestral lineages were once linked by dispersal prior to physical or climatic isolation (Rosen 1978). Low dispersal and vicariance are not mutually exclusive, and distinguishing these two processes in heterogeneous landscapes, especially for poor dispersers, remains therefore a severe challenge. For example, low dispersal (and/or small population size) can give rise to geographic patterns consistent with a phylogeographic break and be mistaken for geographic isolation (Irwin 2002, Kuo and Avise 2005).
The study of Rancilliac and colleagues (2023) is at the heart of this issue. It focuses on a nominal lizard species, the red-tailed spiny-footed lizard (Acanthodactylus erythrurus, Squamata: Lacertidae), which has a wide spatial distribution (from the Maghreb to the Iberian Peninsula), is found in a variety of different habitats, and has a wide range of morphological traits that do not always correlate with phylogeny. The main question is the following: have “the morphological and ecological diversification of this group been produced by vicariance and lineage diversification, or by local adaptation in the face of historical gene flow?” To tackle this question, the authors used sequence data from multiple mitochondrial and nuclear markers and a nested analysis workflow integrating phylogeography, multiple correspondence analyses and a relatively novel approach to IBD testing (Hausdorf & Henning, 2020). The latter is based on regression analysis and was shown to be less prone to error than the traditional (partial) Mantel test.
While this set of methods allowed the partitioning of the effect of isolation by distance and vicariance in shaping contemporary genetic diversity in red-tailed spiny-footed lizards, some of the evolutionary history of this species complex remains blurred by ongoing gene flow and admixture, retention of ancestral polymorphism, or selection. The lack of congruence between mitochondrial and nuclear gene trees once again warns us that proposing evolutionary scenarii based on individual gene trees is a risky business.
References
Hausdorf B, Hennig C (2020) Species delimitation and geography. Molecular Ecology Resources, 20, 950–960. https://doi.org/10.1111/1755-0998.13184
Hickerson MJ, Meyer CP (2008) Testing comparative phylogeographic models of marine vicariance and dispersal using a hierarchical Bayesian approach. BMC Evolutionary Biology, 8, 322. https://doi.org/10.1186/1471-2148-8-322
Irwin DE (2002) Phylogeographic breaks without geographic barriers to gene flow. Evolution, 56, 2383–2394. https://doi.org/10.1111/j.0014-3820.2002.tb00164.x
Kuo C-H, Avise JC (2005) Phylogeographic breaks in low-dispersal species: the emergence of concordance across gene trees. Genetica, 124, 179–186. https://doi.org/10.1007/s10709-005-2095-y
Rancilhac L, Miralles A, Geniez P, Mendez-Aranda D, Beddek M, Brito JC, Leblois R, Crochet P-A (2023) Phylogeographic breaks and how to find them: An empirical attempt at separating vicariance from isolation by distance in a lizard with restricted dispersal. bioRxiv, 2022.09.30.510256, ver. 4 peer-reviewed and recommended by Peer Community in Evolutionary Biology. https://doi.org/10.1101/2022.09.30.510256
Rosen DE (1978) Vicariant Patterns and Historical Explanation in Biogeography. Systematic Biology, 27, 159–188. https://doi.org/10.2307/2412970
Wright, S (1938) Size of population and breeding structure in relation to evolution. Science 87:430-431.
Wright S (1940) Breeding Structure of Populations in Relation to Speciation. The American Naturalist, 74, 232–248. https://doi.org/10.1086/280891
Wright S (1943) Isolation by distance. Genetics, 28, 114–138. https://doi.org/10.1093/genetics/28.2.114