
The difficult task of partitioning the effects of vicariance and isolation by distance in poor dispersers
Phylogeographic breaks and how to find them: Separating vicariance from isolation by distance in a lizard with restricted dispersal
Abstract
Recommendation: posted 13 March 2023, validated 16 March 2023
Pante, E. (2023) The difficult task of partitioning the effects of vicariance and isolation by distance in poor dispersers. Peer Community in Evolutionary Biology, 100634. https://doi.org/10.24072/pci.evolbiol.100634
Recommendation
Partitioning the effects of vicariance and low dispersal has been a long-standing problem in historical biogeography and phylogeography. While the term “vicariance” refers to divergence in allopatry, caused by some physical (geological, geographical) or climatic barriers (e.g. Rosen 1978), isolation by distance refers to the genetic differentiation of remote populations due to the physical distance separating them, when the latter surpasses the scale of dispersal (Wright 1938, 1940, 1943).
Vicariance and dispersal have long been considered as separate forces leading to separate scenarii of speciation (e.g. reviewed in Hickerson and Meyer 2008). Nevertheless, these two processes are strongly linked, as, for example, vicariance theory relies on the assumption that ancestral lineages were once linked by dispersal prior to physical or climatic isolation (Rosen 1978). Low dispersal and vicariance are not mutually exclusive, and distinguishing these two processes in heterogeneous landscapes, especially for poor dispersers, remains therefore a severe challenge. For example, low dispersal (and/or small population size) can give rise to geographic patterns consistent with a phylogeographic break and be mistaken for geographic isolation (Irwin 2002, Kuo and Avise 2005).
The study of Rancilliac and colleagues (2023) is at the heart of this issue. It focuses on a nominal lizard species, the red-tailed spiny-footed lizard (Acanthodactylus erythrurus, Squamata: Lacertidae), which has a wide spatial distribution (from the Maghreb to the Iberian Peninsula), is found in a variety of different habitats, and has a wide range of morphological traits that do not always correlate with phylogeny. The main question is the following: have “the morphological and ecological diversification of this group been produced by vicariance and lineage diversification, or by local adaptation in the face of historical gene flow?” To tackle this question, the authors used sequence data from multiple mitochondrial and nuclear markers and a nested analysis workflow integrating phylogeography, multiple correspondence analyses and a relatively novel approach to IBD testing (Hausdorf & Henning, 2020). The latter is based on regression analysis and was shown to be less prone to error than the traditional (partial) Mantel test.
While this set of methods allowed the partitioning of the effect of isolation by distance and vicariance in shaping contemporary genetic diversity in red-tailed spiny-footed lizards, some of the evolutionary history of this species complex remains blurred by ongoing gene flow and admixture, retention of ancestral polymorphism, or selection. The lack of congruence between mitochondrial and nuclear gene trees once again warns us that proposing evolutionary scenarii based on individual gene trees is a risky business.
References
Hausdorf B, Hennig C (2020) Species delimitation and geography. Molecular Ecology Resources, 20, 950–960. https://doi.org/10.1111/1755-0998.13184
Hickerson MJ, Meyer CP (2008) Testing comparative phylogeographic models of marine vicariance and dispersal using a hierarchical Bayesian approach. BMC Evolutionary Biology, 8, 322. https://doi.org/10.1186/1471-2148-8-322
Irwin DE (2002) Phylogeographic breaks without geographic barriers to gene flow. Evolution, 56, 2383–2394. https://doi.org/10.1111/j.0014-3820.2002.tb00164.x
Kuo C-H, Avise JC (2005) Phylogeographic breaks in low-dispersal species: the emergence of concordance across gene trees. Genetica, 124, 179–186. https://doi.org/10.1007/s10709-005-2095-y
Rancilhac L, Miralles A, Geniez P, Mendez-Aranda D, Beddek M, Brito JC, Leblois R, Crochet P-A (2023) Phylogeographic breaks and how to find them: An empirical attempt at separating vicariance from isolation by distance in a lizard with restricted dispersal. bioRxiv, 2022.09.30.510256, ver. 4 peer-reviewed and recommended by Peer Community in Evolutionary Biology. https://doi.org/10.1101/2022.09.30.510256
Rosen DE (1978) Vicariant Patterns and Historical Explanation in Biogeography. Systematic Biology, 27, 159–188. https://doi.org/10.2307/2412970
Wright, S (1938) Size of population and breeding structure in relation to evolution. Science 87:430-431.
Wright S (1940) Breeding Structure of Populations in Relation to Speciation. The American Naturalist, 74, 232–248. https://doi.org/10.1086/280891
Wright S (1943) Isolation by distance. Genetics, 28, 114–138. https://doi.org/10.1093/genetics/28.2.114
The recommender in charge of the evaluation of the article and the reviewers declared that they have no conflict of interest (as defined in the code of conduct of PCI) with the authors or with the content of the article. The authors declared that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
Part of the analyses were carried out by L.R. using resources in project 2022/22-653 provided by the Swedish National Infrastructure for Computing (SNIC) at UPPMAX, partially funded by the Swedish Research Council through grant agreement no. 2018-05973
Evaluation round #2
DOI or URL of the preprint: https://www.biorxiv.org/content/10.1101/2022.09.30.510256v3
Version of the preprint: 3
Author's Reply, 14 Feb 2023
Dear recommender,
We are once again thankful for you considering our manuscript and suggesting improvements in the text. We have edited the manuscript accordingly.
With best wishes,
Loïs Rancilhac on behalf of the coauthors team
Decision by Eric Pante , posted 10 Feb 2023, validated 13 Feb 2023
, posted 10 Feb 2023, validated 13 Feb 2023
Dear authors,
Thank you very much for thouroughly revising your manuscript in light of the previous comments. I found that all were carefully addressed ; the addition of supplementary files, especially the supplementary tables, will improve the repeatability and perenity of your work. I do not think that it is necessary to send the ms back for review, but I have made a few comments / suggestions directly in the text as an effort to further improve clarity. Could you please have a look at those before I post my recommendation?
Best regards, eric pante
Download recommender's annotationsEvaluation round #1
DOI or URL of the preprint: https://www.biorxiv.org/content/10.1101/2022.09.30.510256v2
Version of the preprint: 2
Author's Reply, 18 Jan 2023
Decision by Eric Pante , posted 15 Dec 2022, validated 15 Dec 2022
, posted 15 Dec 2022, validated 15 Dec 2022
Dear authors,
Thank you very much for submitting your work to PCI Evol Biol. Two reviewers have carefully evaluated your manuscript. I fully agree with them that it is very interesting and worthy of publication, and I commend you on the extent of the data analyses, careful and interpretations, as well as the quality of the writing and presentations. The reviewers have some comments that I think will be quite easy to address. Please make sure that all hyperlinks work in your manuscript (there is an issue with your link to zenodo). I have a comment additional to those of the referees : on line 367 you state that S-Yah has a basal position within Moroccan populations. (1) there is no indication in the methods that the tree is rooted; (2) rephrasing is needed: when using the term 'basal' please explicit to what the clade is basal to ('basal to all other Moroccan clades'?).
Thank you,
eric pante
Reviewed by Kevin Sánchez, 09 Nov 2022
Reviewed by Aglaia (Cilia) Antoniou, 22 Nov 2022
Reviewing the bioRxiv preprint with doi: https://doi.org/10.1101/2022.09.30.510256
Phylogeographic breaks and how to find them: Separating vicariance from isolation by distance in a lizard with restricted dispersal
General comments:
Rancilhac et al. re-analyse available mtDNA and nuDNA datasets of Acanthodactylus erythrurus in an attempt to distinguish the effects of vicariance from those of isolation by distance (IBD). The authors highlight the impacts of the choice of molecular markers (mtDNA vs multiple unlinked nuclear loci) and sampling scheme on the detection of phylogeographic breaks. They focus on Moroccan populations of A. erythrurus having a dataset with comprehensive sampling and analyse multiple markers (mtDNA as well as multi-locus nuclear data) in an attempt to overcome the limitations of incomplete or obscure sampling scheme and the use of a single marker to detect phylogeographic barriers.
The analyses are well conducted investigating the underlying processes that shaped A. erythrurus genetic diversity emphasizing on the nature of breaks (IBD or vicariance) leading the authors to formulate a phylogeographic scenario explaining species present genetic diversity. The analyses partly overlap with those of Miralles et al. 2020 from which data were retrieved with the current study working on a subset of samples (IM clade). However it is not clear that this is a sort of meta-analysis of data produced by another study until the Materials and Methods section. Nevertheless, new analyses have been conducted in an attempt to shed light on different aspects from those of Miralles et al. 2020.
In my opinion, there are some minor issues that the authors need to address in order to make their study and ms clearer and easier for the reader to follow. In terms of language, there are typos throughout the ms while it would be significantly improved if someone proficient in English would make edits (some parts of the manuscript are not clearly written e.g. lines 146-149 rendering the reasoning of the authors not easy to follow). Furthermore in various parts of the ms appropriate references are missing (e.g. lines 146-149).
Specific comments:
Title
I believe that the title is promising of a more theoretical/methodological approach that the one followed in this study (e.g. of proposing a new way of discriminating between IBD and phylogeographic barriers underlying vicariance events). Therefore I believe that the title is not an accurate description of the conducted study and does not clearly reflect its contents.
Abstract
The abstract is concise and presents the main findings of the study.
Introduction
Introduction explains the motivation and the questions that this study attempts to address. However it would be important to make clear that that this is a sort of meta-analysis study and refer to the importance of meta-analysis.
Materials and Methods
At the Materials and Methods section, there is a lack of justification why mtDNA data (displaying the higher resolution groupings) were not used to test IBD in a comparative framework with those of nuDNA. Furthermore, (L186) it is not clear which samples were used in the study i.e. Miralles et al. 2020 molecular analyses involved 128 samples and not 392. Furthermore it appears that the study focused on IM clade of Miralles et al. 2020 and not on the Moroccan populations as stated in L195. If this is not the case then the authors need to justify why Moroccan samples from Tizi n’ Tichka and Siroua (WHA in Miralles et al. 2020), Isli and Tislit (EHA in Miralles et al. 2020) were not included in the analyses.
L200 “The samples were grouped based on their mitochondrial lineages” in order to run PHASE to infer individual genotypes. Please provide a justification why you preferred to run PHASE on each group separately. Given the drawbacks of mtDNA in defining evolutionary units (also stated in the introductory sessions by the authors) I find this rather problematic.
L234-235 I am not sure what the authors mean by “conducting independent analyses of the same data” since STRUCTURE performs “independent” analyses for each K and each replicate run within each K. Were the resulting solutions bi- or multi-modal? Does any make biological sense? Do the different groups/clusters contain samples with high membership coefficients (q-values)? This in my opinion is important information that needs to be communicated.
L234-239 this belongs to the Results section.
L257 “an alternative approach” Please clarify to which approach(es) this constitutes an alternative
L258 Maybe here it is Factorial Correspondence Analysis (FCA)?
L274 the justification of this decision is lacking
Results
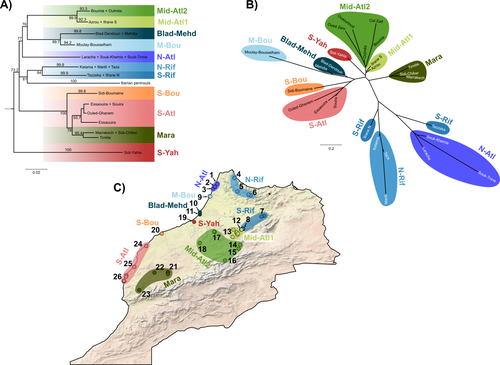
L351-363 The description of the phylogenetic tree in this section is not in agreement with the one depicted in Figure 1. Please make the appropriate changes to Figure1A as to indicate the >90 support of the 11 clades.
Figure1C: it would be helpful for the reader to indicate on the map the three deeper mtDNA groups (e.g. by encircling them). Furthermore adding the group codes to the mtDNA tree and nuDNA network will also be helpful. There is probably a shift in the numbers of the localities (S-Yah is 19 at the Figure and 20 at the Table, S-Bou is 20 at the Figure and 21 at the table etc.) with number 27 missing from the Figure.
L426-427 this is not the case in Figure 1B where Mara is closer to the remaining than to Rif, N-Atl groups.
Discussion
It is very important to infer the results in the light of the assumptions made by the different approaches employed (e.g. STRUCTURE and IBD, Phylogenies and the level of differentiation in the dataset, IBD and the assumption of linear relationship of genetic and geographic distances). I would like the authors to elucidate more on the fact that there are geographically distant sites that belong to the same group as well as site in close geographical proximity that belong to different groups e.g. by comparing environmental conditions of the respective areas.
L507-511 couldn’t these results be justified by the attributes of the analysed nuclear markers (e.g. being under selection)?
L505-507 and L513 attention must be given to the fact that STRUCTURE should be avoided when IBD holds since such datasets do not conform precisely to the structure model while it is difficult to infer K.
There are too many redirections to the “next section” (e.g. L543-544, 546-547, 560-561) that make it difficult for the reader to follow.
L558 phylogenetic analyses cannot by any means be considered as simple or simpler approaches to the ones designed to test IBD patterns. The two aforementioned approaches are better suited for addressing different questions focusing at different levels of divergence although phylogenetic methods can be considered as more flexible covering a wider range of diversification (coalescence, yule etc).
L563 also genome-wide
L565-570 this contradicts somehow the statements made at L559-561.
L641 Do those breaks reflect present distinct environmental conditions?
L642-644 Is there any information/hypothesis on which areas faced the harshest conditions?










