
Trade-offs in fitness components and ecological source-sink dynamics affect host specialisation in two parasites of Artemia shrimps
Trait-specific trade-offs prevent niche expansion in two parasites
Abstract
Recommendation: posted 02 December 2019, validated 09 December 2019
Guillaume, F. (2019) Trade-offs in fitness components and ecological source-sink dynamics affect host specialisation in two parasites of Artemia shrimps. Peer Community in Evolutionary Biology, 100203. https://doi.org/10.24072/pci.evolbiol.100203
Recommendation
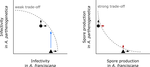
Ecological specialisation, especially among parasites infecting a set of host species, is ubiquitous in nature. Host specialisation can be understood as resulting from trade-offs in parasite infectivity, virulence and growth. However, it is not well understood how variation in these trade-offs shapes the overall fitness trade-off a parasite faces when adapting to multiple hosts. For instance, it is not clear whether a strong trade-off in one fitness component may sufficiently constrain the evolution of a generalist parasite despite weak trade-offs in other components. A second mechanism explaining variation in specialisation among species is habitat availability and quality. Rare habitats or habitats that act as ecological sinks will not allow a species to persist and adapt, preventing a generalist phenotype to evolve. Understanding the prevalence of those mechanisms in natural systems is crucial to understand the emergence and maintenance of host specialisation, and biodiversity in general. In their study "Trait-specific trade-offs prevent niche expansion in two parasites", Lievens *et al.* [1] report the results of an evolution experiment involving two parasitic microsporidians, *Anostracospora rigaudi* and *Enterocytospora artemiae*, infecting two sympatric species of brine shrimp, *Artemia franciscana* and *Artemia parthenogenetica*. The two parasites were originally specialised on their primary host: *A. rigaudi* on *A. parthenogenetica* and *E. artemiae* on *A. franciscana*, although they encounter both species in the wild but at different rates. After passaging each parasite on each single host and on both hosts alternatively, Lievens *et al.* asked how host specialisation evolved. They found no change in specialisation at the fitness level in *A. rigaudi* in either treatment, while *E. artemiae* became more of a generalist after having been exposed to its secondary host, *A. parthenogenetica*. The most interesting part of the study is the decomposition of the fitness trade-off into its underlying trade-offs in spore production, infectivity and virulence. Both species remained specialised for spore production on their primary host, interpreted as caused by a strong trade-off between hosts preventing improvements on the secondary host. *A. rigaudi* evolved reduced virulence on its primary host without changes in the overall fitness trad-off, while *E. artemiae* evolved higher infectivity on its secondary host making it a more generalist parasite and revealing a weak trade-off for this trait and for fitness. Nevertheless, both parasites retained higher fitness on their primary host because of the lack of an evolutionary response in spore production. This study made two important points. First, it showed that despite apparent strong trade-off in spore production, a weak trade-off in infectivity allowed *E. artemiae* to become less specialised. In contrast, *A. rigaudi* remained specialised, presumably because the strong trade-off in spore production was the overriding factor. The fitness trade-off that results from the superposition of multiple underlying trade-offs is thus difficult to predict, yet crucial to understand potential evolutionary outcomes. A second insight is related to the ecological context of the evolution of specialisation. The results showed that *E. artemiae* should be less specialised than observed, which points to a role played by source-sink dynamics on *A. parthenogenetica* in the wild. The experimental approach of Lievens *et al.* thus allowed them to nicely disentangle the various sources of constraints on the evolution of host adaptation in the *Artemia* system. **References** [1] Lievens, E.J.P., Michalakis, Y. and Lenormand, T. (2019). Trait-specific trade-offs prevent niche expansion in two parasites. bioRxiv, 621581, ver. 4 peer-reviewed and recommended by PCI Evolutionary Biology. doi: [10.1101/621581](https://dx.doi.org/10.1101/621581)
The recommender in charge of the evaluation of the article and the reviewers declared that they have no conflict of interest (as defined in the code of conduct of PCI) with the authors or with the content of the article. The authors declared that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
no declaration
Evaluation round #1
DOI or URL of the preprint: https://doi.org/10.1101/621581
Version of the preprint: 2
Author's Reply, 26 Nov 2019
Decision by Frédéric Guillaume , posted 08 Jul 2019
, posted 08 Jul 2019
Dear authors,
After receiving three reviews and having assessed your work, I think that your manuscript would greatly benefit from some revisions. All reviewers agree that your work is original and presents interesting results on the evolution of host specialization. I particularly like the trait-based approach that you used and find the overall analysis sound and well conducted. Most reviewers, including myself, agree with your main conclusions. I am convinced that your work will reach a broad audience interested in the role of trade-offs in the evolution of specialization.
The reviewers ask for some clarifications in the presentation of the study system and of the results. I join them in asking you to revise your manuscript and to carefully address the issues raised during this first round of reviews. A revised version will be suitable for a recommendation.
The main reviewers' comments can be summarized as follows:
- please comment on a possible role of inter-parasite competition during infection of the hosts, provided that multi-infections do occur?
- clarify what is the main mode of genetic adaptation in your experiment: from de-novo mutations or standing variation? and what it implies for the evolutionary dynamics of the traits during the experiment.
- better discuss alternative explanations for the apparent lack of adaptive evolution of spore production; is it a strong trade-off or a lack of genetic variation in that trait? can we interpret this lack of evolution as a negative result?
- enhance the presentation of the ecology of the system and the general literature on the topic.
To those, I am adding a few of my own points:
- please clarify if A. parthenogenetica is an obligate host of A. rigaudi, as suggested by your comment on p4 lines 73-74 ("A. rigaudi has much higher fitness in A. parthenogenetica and cannot persist without this host in the field"); how does this affect A. rigaudi's survival in the lab and the results of your experiment, esp. when A. rigaudi is exposed to the alternate host only?
- similarly, if survival of A. rigaudi depends on presence of A. parthenogenetica, how does the parasite persist in the winter when A. p. is absent? (as per page 14 line 364: "A. parthenogenetica are only present from late spring to fall").
- furthermore, it seems that the two parasites don't have the same ecology, and may not co-occur in nature (p14 line 369); all these details appear late in the manuscript. Please provide a clearer and more complete picture of the ecology of the two species earlier in the manuscript, in the Methods section preferably.
- what is the role of phenotypic plasticity in your experiment? can you discuss your results in terms of evolution of norms of reaction instead of only mean trait values? How plastic are the traits you measured? your results clearly present cases of evolution of reaction norms, with maybe mostly changes in intercepts (mean) than slopes (beside maybe virulence). Please comment.
I would be grateful if you could comment on the points raised here and by the reviewers, providing arguments when you don't agree. Please provide a point-by-point answer with clear reference to the main text to speed up the assessment of your revised version. Thanks!
Reviewed by Seth Barribeau, 01 Jul 2019
Lievens et al. present an impressive body of work. The topic is interesting and the approach is sensible. Experimental evolutions studies can be challenging, and the effort here should be commended. I do however have some issues, listed below. Among those, I think the most important involve the interpretation of the results. First, if I understand correctly, the infectious exposure came from diverse stock, it then seems likely that any adaptation was selection on standing genetic variation rather than mutations. Second, I have trouble wrapping my head around the explanation that the absence of adaptation to experimental conditions represents strong trade-off, as opposed to simply a negative result.
Line-by-line comments
L66: on -> in L68: "in host use limit the evolution of specialization of two naturally occurring parasite" Previously talk about barriers to generalism. Here inverted. I get that these are two sides to the same coin, but good to be consitant to ensure readers follow. Similar around L78
L97: what is meant by suboptimal virulence? Too high or too low?
L102: Genetic diversity of parasite stocks unknown, but collected from various sites
parag around 115: italics for Latin names
L125: "we did not control the number of spores that were transmitted from one group of hosts to the next. Thus, the size of the inoculum and of the microsporidian population in all passages after P1 were dependent on the infection dynamics that developed within each replicate line." Is this a problem? different doses selection for what then?
L150 missing ref
L171: surviving and revived lines... what does that mean? passsage 10 vs 6? Figured it out later, but could do with clarifying first use of 'revived'
L181: only includes infected individuals in survival analysis. How many/what proportion were excluded because they were exposed but not infected?
Results
Pretty complicated results. Not unsurprisingly. Lots of stuff going on.
L252: A. rigaudi is most virulent when assayed on A. parthenogenetica if passaged on other host. Suggests adaptation to host sp. and perhaps suboptimal virulence on the other. This is an important result and a figure here would be useful. This is shown in Fig4 but figure not mentioned in text here.
Same for L274
L257: "but infected hosts did not die faster than controls" unclear here what is control. Presumably all were infected?
Discussion
L285-6: "infectivity readily evolved towards generalism" This doesn't appear true. E. aremiae did evolve greater infectivity on A.parthenogenetica but A. rigaudi did not. Similarly, "virulence played a minor role" doesn't seem quite true either as A. rigaudi evolved reduced virulence on A. parthenogenetica when tested on that host. Figure 4.
L286-288: "Our results are consistent with a strong trade-off acting on spore production and a weak trade-off on infectivity, and suggest that spore production is the key trait preventing the evolution of generalism in this system."
It is pushing it a bit to interpret no adaptation to the experimental evolution treatment as strong trade-off limiting the evolution of spore production.
Paragraph starting at L319: As genetic diversity of the initial infectious dose is unknown but taken from multiple source populations (L106) I would have thought that the most parsimonious explanation in the absence of genetic evidence is that there was selection upon standing genetic variation rather than accumulation of mutations of any flavour.
"It is worth noting that our conclusions would have been very different if we had not measured the parasites’ traits separately. Based on the overall fitness (Fig. 5), we would have concluded that A. rigaudi was unable to adapt to its mismatched host, while E. artemiae was able to evolve towards generalism after exposure to A. parthenogenetica. This would have suggested that the two parasites had asymmetrical fitness trade-offs between hosts: a strong trade-off for A. rigaudi, and a weaker trade-off for E. artemiae."
I certainly agree that measuring different components of parasite biology is informative. But I may have missed something here. This sounds precisely like the argument given by figure 5. That E. artemiae has weaker trade-offs, hence the blue arrow.
Fig4: gray is hard to make out. Also not obvious the star system. eg. stars over curly brackets, does this say that the values within this group are sig different from one another, or from teh other group in curly brackets. Looking at the figure I would have said within group but the values look too similar to be different from one another. I would recommend add square brackets to clarify that the stars refer to between host species.
Based on supplemental materials, there should be a strongly significant difference between virulence of E. artemiae in the different hosts (X=58.1, p<0.0001), but is not given in figure. Nor do the virulence values look very different in the different hosts.
Suppl
fig1: why so few evolved lines (dashed, hollow points, '+' signs?) - only three of the plots have one of those.
https://doi.org/10.24072/pci.evolbiol.100203.rev11Reviewed by Anne Duplouy, 14 Jun 2019
Lievens et al. provide here a study testing and discussing the evolution of niche specialization in a parasite, using an experimental evolution approach. After 10 generations of passaging separately two parasites on either their preferred host/occasional host/or both(alternatively between passages), they collect data on parasite spore production, infectivity of parasite (define as % of hosts infected during assay) and parasite virulence (defined as ratio of time until host death compared to control hosts) of the evolved lines on the two types of hosts.
The hosts species are A. franciscana and A. parthenogenetica - The parasites are microsporidia E. artemiae and A. rigaudi. The study shows that all 6 evolved lines maintained a higher fitness on their preferred host species. This results is mostly driven by a strong variation in spore production between parasite-host associations. Despite 10 generations of habituation to their occasional hosts A. franciscana , the A. rigaudi parasites evolved lines still produced as much spores as the A. rigaudi lines that have evolved on A. parthenogenetica, when both lines were put in contact with A. franciscana in a last essay. (And the other way around for E. artemiae evolved lines). The authors thus suggest that spore production is the barrier preventing the evolution of generalist strategy in these host/parasite systems. Additionally, E. artemiae evolved lines show variations in their fitness when put in contact to A. parthenogenetica in the last essay, while the three evolved lines show similar fitness. The authors here suggest that E. artemiae can evolve a more generalist strategy, while A. rigaudi can not.
In brief, the authors argue that a weak trade off between the ability of a parasite to successfully infect host1 and its ability to infect host2 could evolve a more generalist strategy. In contrast, if the trade offs are strong, the parasite is most likely to remain a specialist of host1. I believe such results might have important implications for the study of parasitism, highlighting potential constraints to their spread and evolution in natural environments.
I have little comments on this research article. I would simply ask the authors to more rapidly describe the type of trade-offs they are considering. I am an Ecologist and Evolutionary Biologist. My first idea of a trade-off is between two different traits pulling on opposite direction, here the trade-offs refer to one unique trait but pulled to it's opposite extremes in the two host environment. It might help if this is made clear from the abstract.
L146-150: This is not 100% informative. I would have prefer to have a clear explanation of when these tests were necessary and used; rather than have to guess when you actually used them.
Figure 2: I wonder if you could add information on which host species are each passage of the 'Alternating host' line.
Figure 4&5: to make it clear maybe, and stand alone figure; potentially add 'Evolved line of A.rigaudi' and 'evolved line of E. artemiae' at the top of figures. And to keep consistent with your text maybe 'Final assay host' at the bottom of your graph.
On L.248-249: You write: 'No infection was detected for the line---Replicate 1, so it was excluded from further analyses. This particular replicate is however included in Table 3, and is not counted as 'lost', Did I read this table wrongly?
Could you also discuss the fact that in the field, those parasites are not occurring on their own on those hosts, and competition between parasite is likely to influence each of the traits you have been studying here. Is there any data on whether one or the other parasite is more competitive? Or is there anything on other potential hosts or 'reservoirs' for these Microsporidians in their natural environments/regions where you collected them?
Thanks
https://doi.org/10.24072/pci.evolbiol.100203.rev12









