
Evolution of cross-tolerance: a mechanism to cope with climate change?
 based on reviews by Marina Stamenkovic-Radak and 1 anonymous reviewer
based on reviews by Marina Stamenkovic-Radak and 1 anonymous reviewer
Cross-tolerance evolution is driven by selection of heat tolerance in Drosophila subobscura
Abstract
Recommendation: posted 24 May 2024, validated 31 May 2024
Simões, P. (2024) Evolution of cross-tolerance: a mechanism to cope with climate change? . Peer Community in Evolutionary Biology, 100709. https://doi.org/10.24072/pci.evolbiol.100709
Recommendation
Understanding how populations evolve under thermal stress and how this process shapes the response of other stress responses is an important research topic in the context of thermal adaptation and climate change. In a thermal experimental evolution study in Drosophila subobscura, Castañeda (2024) addressed the correlated responses to selection for increasing knockdown temperature in different resistance traits, either directly related to thermal stress (e.g. knockdown time at different temperatures and CTmax) or not (e.g. desiccation and starvation resistance).
The author found that the evolution of higher knockdown temperature did in fact lead to correlated responses in other stress traits. While such correlations might be expected for the thermal stress traits measured (knockdown time and CTmax), it was perhaps less expectable for desiccation and starvation resistance. However, the general occurrence of correlated evolutionary responses between stressors has been previously described, namely in Drosophila (e.g. see Bubliy and Loeschcke 2005), pointing to a possible genetic link between distinct (thermal) stress traits.
There are however some features that make the findings of this study rather appealing. First, the evidence that the correlated stress responses depend on the intensity of thermal selection (i.e. the warming rate) and on the sex of the organisms. Second, correlated patterns of both desiccation and starvation resistance highlight the possibility of the evolution of a cross-tolerance response, which might positively impact on population ability to evolve under sustained stressful environments (Rodgers and Gomez Izasa 2023). However, it is important to point out that the correlated patterns between these two resistance traits (desiccation and starvation) were not exactly consistent. In fact, the negative correlated response observed for female starvation resistance is thought provoking and argues again a general scenario of cross-tolerance.
While these findings are a step forward for a more multifaceted understanding of thermal adaptation in the context of stressful environments, they also highlight the need for further studies of thermal adaptation namely 1) addressing the underlying physiological and genomic mechanisms that link male and female heat tolerance and the response to other stress resistance traits (namely starvation resistance); 2) testing the extent to which cross-resistance patterns can be generalized to different thermal selection contexts and populations.
In addition, this study also opens new questions considering the scope of correlated evolution to other stress traits, that might be relevant in diverse ecological scenarios. For instance, does selection towards higher heat resistance lead to correlated evolution of cold resistance? And under which circumstances (e.g. different heat selection intensities)? In fact, the occurrence of a positive (or negative) correlation cold and heat stress responses is a topic of high interest, with relevant ecological implications particularly considering the increased thermal fluctuations in natural environments because of climate warming. Cross-tolerance between cold and heat stress responses has been described (Singh 2022, Rodgers and Gomez Izasa 2023). On the other hand, negative correlations (i.e. trade-offs) between these stress traits (Stazione et al. 2020; Schou et al 2022) can impact negatively on populations’ ability to withstand thermal variability.
As climatic changes proceed leading to increasing environmental variability, empirical studies such as that of Castañeda (2024) are critical in the pursue for a multivariate perspective on trait evolution in scenarios of climate change adaptation. Understanding how tolerance to different environmental stressors may evolve and which factors can act as drivers of that variation will ultimately enable better forecasts of climate change effects on biodiversity in nature.
References
Castañeda, LE. Cross-tolerance evolution is driven by selection on heat tolerance in Drosophila subobscura. Biorxiv, ver. 4 peer-reviewed and recommended by Peer Community in Evolutionary Biology (2024). https://www.doi.org/10.1101/2023.09.05.556367
Bubliy, OA, Loeschcke, V. Correlated responses to selection for stress resistance and longevity in a laboratory population of Drosophila melanogaster. J Evol Biol. 18(4):789-803 (2005). https://www.doi.org/10.1111/j.1420-9101.2005.00928.x.
Rodgers, EM, Gomez Isaza, DF. The mechanistic basis and adaptive significance of cross-tolerance: a 'pre-adaptation' to a changing world? J Exp Biol. 226(11):jeb245644 (2023). https://www.doi.org/10.1242/jeb.245644.
Schou, MF, Engelbrecht, A, Brand, Z, Svensson, EI, Cloete, S, Cornwallis, CK. Evolutionary trade-offs between heat and cold tolerance limit responses to fluctuating climates. Sci Adv. 8(21):eabn9580 (2022). https://www.doi.org/10.1126/sciadv.abn9580.
Singh, K, Arun Samant, M, Prasad, NG. Evolution of cross-tolerance in Drosophila melanogaster as a result of increased resistance to cold stress. Sci Rep. 12(1):19536 (2022). https://www.doi.org/10.1038/s41598-022-23674-z.
Stazione, L, Norry, FM, Gomez, FH, Sambucetti, P. Heat knockdown resistance and chill-coma recovery as correlated responses to selection on mating success at high temperature in Drosophila buzzatii. Ecol Evol. 10(4):1998-2006 (2020). https://www.doi.org/10.1002/ece3.6032.
The recommender in charge of the evaluation of the article and the reviewers declared that they have no conflict of interest (as defined in the code of conduct of PCI) with the authors or with the content of the article. The authors declared that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
FONDECYT 1140066
Evaluation round #3
DOI or URL of the preprint: https://www.biorxiv.org/content/10.1101/2023.09.05.556367v3
Version of the preprint: 3
Author's Reply, 23 May 2024
Decision by Pedro Simões , posted 17 May 2024, validated 20 May 2024
, posted 17 May 2024, validated 20 May 2024
I thank the author for this revised version. I will gladly write a recommendation for this article once some few remaining (minor) issues are addressed:
- Line 247: change to “linear mixed model”
- Line 329-331: Clarify that this text refers to a different, additional analysis
- Lines 334-335: This interaction is not seen in Table S5, right?
- Line 349: Correct to “starvation resistance”
Evaluation round #2
DOI or URL of the preprint: https://doi.org/10.1101/2023.09.05.556367
Version of the preprint: 2
Author's Reply, 13 May 2024
Decision by Pedro Simões , posted 16 Apr 2024, validated 17 Apr 2024
, posted 16 Apr 2024, validated 17 Apr 2024
I thank the author for his effort in replying to all the questions / comments raised in the first round of revision. My assessment is that the manuscript has improved. There are still some points raised by one reviewer - mostly concerning the statistical approach - that should be considered, in order to improve the clarity of the methodology used.
Best regards,
Pedro Simões
Reviewed by anonymous reviewer 1, 15 Apr 2024
I have read the revision of the paper - it greatly improved, but I have some further suggestions that might improve the statistical presentation of results.
(1) I find it confusing at what stages the slow vs fast ramping regime was analysed in one analysis, and when these were analysed separately. From what I understand - the first analysis of knockdown times was done separately, and then at what stage/in which analyses the slow/fast selected lines were pooled? Does "Selection" fixed effect always mean slow vs fast, or does it mean selected vs control? It should be clearer and the naming of factors should leave no space for ambiguity here...
(2) Random effects - from what I see you had 1 random effect (line) - how was it tested in lme4 using LRT? This package does not allow for fitting a model without random effects, which would be needed to compare it with the one with the line effect. Also - you report only 1 result of LRT - was it the only analysis where random effect were used?
(3) I think analysing all static assay temperatures is superfluous (not to mention the multiple comparisons it generates, I think such results, if not corrected for false-positive discovery rate inflation (e.g., via p.adjust() in R), may be anticonservative). What I suggest is sticking to the knockdown time ~ temp relationship for each fly and line, and using a mixed effect ANCOVA to test hypotheses. In such analysis, the intercept differences would reflect changes in knockdown time, and changes in slope (interaction of temperature and selection regime) would reflect potential effects of selection on CT_max; such a model would also allow for random slopes (variation in t~temp slope variation across replicate lines).
Minor
L18-20 There's something wrong with the word order in this sentence
L111-112 What are the "confounding effects" here? This prediction is not clear at all.
L143 at a rate, not TO a rate (similar typo in several other places)
L234 glmer, not glm?
L346-347 In what species and/or study type?
L358 assay, not ASSAYED
I suggest one more read looking for other typos or leftovers from previous version.
https://doi.org/10.24072/pci.evolbiol.100709.rev21Evaluation round #1
DOI or URL of the preprint: https://doi.org/10.1101/2023.09.05.556367
Version of the preprint: 1
Author's Reply, 15 Feb 2024
Decision by Pedro Simões , posted 24 Nov 2023, validated 24 Nov 2023
, posted 24 Nov 2023, validated 24 Nov 2023
Thank you for submitting your study for recommendation in PCI Evol Biol. The study of how heat tolerance evolves as a function of stress intensity is a very relevant question. It is also important to understand how changes in heat tolerance affect other relevant traits in a correlated manner. I have asked two reviewers to comment on your manuscript, that you can find attached below. Both reviewers are generally positive about the manuscript although asking for several revisions to be made. Based on the reviewers comments I highlight the need for a flow-chart/image associated with each key stage of your experiment, the accessibility of data as well as to consider their comments on the interpretation / discussion of your results. I would also include the need for further discussion concerning the starvation resistance patterns, which I found particularly surprising as well as some additional clarifications (see also my comments below). Considering the above, I encourage you to resubmit a revised version of your manuscript carefully considering all points raised.
Below I include some additional comments:
Lne 42 – “..these traits” – heat tolerance? Please be more specific.
Line 58 – ref. 28 is incorrectly attributed.
Lines 81-83 - the work by Bubliy and Loeschcke 2005 is an important reference in this context.
Lines 84-87 – briefly explain the selection lines which are the basis of this study and/or connect them with refs. 26 and 27. The selection lines are not explained in the context of the present study (only mentioned in the expectations) and are not integrated with previous studies (refs 26 and 26).
Line 101. Italicize “D. subobscura”
Line 119. “…four population cages assigned to different selection regimes”
Line 159. 5 flies from each sex? how many vials were used per replicate?? this is needed to obtain the total sample size
Line 172. “response of the knockdown temperature” seems counterintuitive as this is not a trait that can evolve/respond …consider changing to “knockdown resistance”?
Line 228 – This reference is incorrectly attributed, please carefully check the reference list.
Line 309-310. This sentence has a weird structure, please correct.
Line 314. I would be more cautious here as for starvation resistance you do not have such a positive cross-resistance pattern (only for males in slow ramping)...
Lines 330-331. Because the assay is longer right? Please clarify
Line 373. This sentence has a weird structure, please correct.
Line 381-382. I do not follow this reasoning...wouldn't this mean that selection would then be stronger in males than in females?
Line 387-390. The decline in starvation resistance in the selected lines is not intuitive and should be more clearly discussed. Could it be related with changes in other traits, namely with the previous reported changes in fecundity (ref 27)? In this context, I believe Rogell et al (2014) https://doi.org/10.1111/1365-2435.12179 is a study to consider, as the authors discuss the processes underlying sex-specific differences in populations under thermal selection.
Please cite Table 2 in the main text.
Line 627-629. “Average value for each replicated population” – wouldn’t this mean three data points for each thermal regime (considering that each has 3 replicate lines)? Or are you plotting the average of the three replicates? Please clarify.
Reviewed by anonymous reviewer 1, 22 Nov 2023
I read the paper with great interest - it is an nice experiment that targets the artificial (co)evolution of traits associated with thermal sensitivity in the fruit fly. However, I have some comments and questions that may be valid for the interpretation of the results.
1) So you created 100 isofemale lines - but then their identiy is largely lost, where they maintained (i.e., 100 lines, then each split into 3 reps and 4 groups) throughout the experiment? It is confusing as it is very difficult to find ones way through the maze of N values, and how different sample sizes come together to describe the power of the study. Please provide: a flow-chart/image of your experiemnt, where at each key stage you specify the number of enclosures (bottles/cages/vials), number of lines and number of individual flies breeding/participating in assays.
2) Also on the question of lines and replicates: what where the results for random effects? Variance estimates etc - this information is entirely missing from the paper. Can you provide open data and code (I strongly suggest thi is available before next review)? Where there changes in variance components between selection regimes?
3) Presentation of results: please provide CIs for all relevant estimates (instead of SE, it emphasizes interval-based conclusions). Also - what are LTR_5 etc in the desication results section? Why are some presented as just LTR and some with a chi-squared statistic?
4) Is it possible that actually selection you imposed was also selecting for desication resistance (so dessication resistance would not evolve in correlation but under direct selection?) I mean - your selection procedure could target both individual's ability to resist thermal stress but also to survive dessication. Would that be a valid explanation?
5) Since it is a selection experiment: I miss basic estimates (in the founding population) of standing genetic variance in traits that were then studied as evolving under selection. Do you have such estimates? Would isofemale lines be inbred enough to provide such estimates? Would genetic variance in base population be enough to justify the observed response to directional selection?
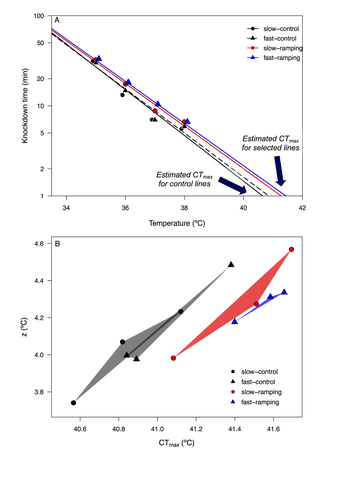
6) What is the interpretation of the polygon are in Fig 2B? You refer to it as "area occupied in the thermal sensitivity landscape" - but is it just a measure of error in linear relationship between z and CT_max? Are CT_max and z expected to be thermally related? If so - this area measure would largely be a statistical byproduct of uncertain values...
The text needs some typo fixing and English editing.
https://doi.org/10.24072/pci.evolbiol.100709.rev11Reviewed by Marina Stamenkovic-Radak, 11 Nov 2023
Adaptive response to global warming, although species specific, generally depends on the population genetic variability and thermal stress intensity. The Author of this paper clearly emphasize the significance and background of research within that topic in introduction. Natural populations are exposed to multiple environmental stressors, and it is known that increased tolerance to one stressor can boost tolerance to another. The major controbution of the present study is that focus is on the effect of variable heat stress intensity on the correlated responses of resistance traits, such as the desiccation and starvation resistance in a Chilean population of D. subobscura. The experimental design is given clearly, with sufficient details, including descripiton of methods performed. Statistical analyses are appropriate and I do not find any missing interpeatation in the results. All Tables and Figures are readable and clear.
The conclusions of this study are adequately supported by the results. The obtained results show the correlated response to thermal stress selection for the studied resistance traits in D.subobscura under the goven experimental design, which demonstrates that the evolutionary response to tolerate higher temperatures also confers the capacity to tolerate other stress such as desiccation and starvation. As Author correctly states, these correlated responses depended on the intensity of thermal selection and sex, which could limit the capacities to transfer these findings to natural scenarios. However, it does not downplay the the value of experimental evolutionary approach to explore and to understand the adaptive responses of natural populations to global warming.
The chosen species D. subobscura has been proven to be ideal to study „contemporary evolution“ as natural experiment, since it has adapted to New world from Palearctic and spreaded quickly by adapting to new enviroments. In that respect, my only remark (suggestion) for this paper is that discussion should take account the results of the genetic variabilty bacground and possible causes of evolutionary and cros evolution response obtained in this particular species. In D. subobacura, the inversion coadaptation studies related to thermal selection have been studied throughly in the light of climatic change. Thus, some of the results, relevant for this research I think should be commented. The Author says that further evidence is needed such as quantitative genetic or genome-wide analysis studies to elucidate the genetic basis of the cross-tolerance evolution in D. subobscura, but I think that they already exist and could shed some light to the results based on the hypothesis in this paper. This species has unique inversion polymorphism, and some of the gene arrangements within individual chromosomes have been proved as useful and informative markers for adaptaion under thermal stess.
https://doi.org/10.24072/pci.evolbiol.100709.rev12









