
RIVERO Ana
- MIVEGEC, CNRS, Montpellier, France
- Evolutionary Applications, Evolutionary Ecology, Evolutionary Epidemiology, Life History, Phenotypic Plasticity
- recommender
Recommendations: 2
Review: 1
Recommendations: 2
An interaction between cancer progression and social environment in Drosophila
Cancer and loneliness in Drosophila
Recommended by Ana Rivero based on reviews by Ana Rivero and Silvie HuijbenDrosophila flies may not be perceived as a quintessentially social animal, particularly when compared to their eusocial hymenopteran cousins. Although they have no parental care, division of labour or subfertile caste, fruit flies nevertheless exhibit an array of social phenotypes that are potentially comparable to those of their highly social relatives. In the wild, Drosophila adults cluster around food resources where courtship, mating activity and oviposition occur. Recent work has shown not only that social interactions in these clusters condition many aspects of the behaviour and physiology of the flies [1] but also, and perhaps more unexpectedly, that social isolation has a negative impact on their fitness [2].
Many studies in humans point to the role of social isolation as a source of stress that can induce and accelerate disease progression. The ultimate proof of the connection between social interaction and disease is however mired in confounding variables and alternative explanations so the subject, though crucial, remains controversial. With a series of elegant experiments using Drosophila flies that develop an inducible form of intestinal cancer, Dawson et al [3] show that cancer progresses more rapidly in flies maintained in isolation than in flies maintained with other cancerous flies. Further, cancerous flies kept with non-cancerous flies, fare just as badly as when kept alone. Their experiments suggest that this is due to the combined effect of healthy flies avoiding contact with cancerous flies (even though this is a non-contagious disease), and of cancerous flies having higher quality interactions with other cancerous flies than with healthy ones. Perceived isolation is therefore as pernicious as real isolation when it comes to cancer progression in these flies. Like all good research, this study opens up as many questions as it answers, in particular the why and wherefores of the flies’ extraordinary social behaviour in the face of disease.
References
[1] Camiletti AL and Thompson GJ. 2016. Drosophila as a genetically tractable model for social insect behavior. Frontiers in Ecology and Evolution, 4: 40. doi: 10.3389/fevo.2016.00040
[2] Ruan H and Wu C-F. 2008. Social interaction-mediated lifespan extension of Drosophila Cu/Zn superoxide dismutase mutants. Proceedings of the National Academy of Sciences, USA, 105: 7506-7510. doi: 10.1073/pnas.0711127105
[3] Dawson E, Bailly T, Dos Santos J, Moreno C, Devilliers M, Maroni B, Sueur C, Casali A, Ujvari B, Thomas F, Montagne J, Mery F. 2017. An interaction between cancer progression and social environment in Drosophila. BiorXiv, 143560, ver. 3 of 19th September 2017. doi: 10.1101/143560
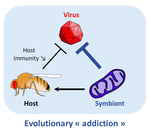
Addicted? Reduced host resistance in populations with defensive symbionts
Hooked on Wolbachia
Recommended by Ana Rivero and Natacha KremerThis very nice paper by Martinez et al. [1] provides further evidence, if further evidence was needed, of the extent to which heritable microorganisms run the evolutionary show.
Wolbachia is an ubiquitous endosymbiont of arthropods who has been recently shown to protect its hosts against viral infections. Here, Martinez et al. are able to show that this multifaceted heritable symbiont weakens selective pressures induced by viruses on host immune genes. In a series of very elegant experiments, Wolbachia-infected and Wolbachia-free populations of D. melanogaster were exposed to Drosophila C virus (a natural, and highly virulent Drosophila pathogen). At the end of a 9-generation artificial selection protocol with DCV, resistance against DCV increased in flies, both in the presence and absence of Wolbachia. Wolbachia-infected flies were still substantially more resistant to DCV viruses than their Wolbachia-free counterparts. Crucially, however, the frequency of the pastrel resistant allele (a key immune gene for DCV resistance) was significantly lower in the Wolbachia-infected lines. As a consequence, when the DCV-evolved lines were treated with antibiotics to cure them from the bacterial infection, the lines who had evolved with Wolbachia tended to be more susceptible to the virus than their uninfected counterparts.
In other words, infection by protective heritable symbionts can affect how selection acts on the host's nuclear-based resistance, effectively rendering it dependent on its symbiont for the fight against pathogens.
But the interest of these results may not be simply academic. The protective qualities of Wolbachia against a range of pathogens have opened up the exciting possibility of transferring these bacteria to mosquito vectors of key human diseases such as dengue or malaria. The long term evolutionary potential for these novel Wolbachia-host interactions has, however, been little explored. Either the Wolbachia, the pathogen or, as shown here, the host, could evolve in more or less predictable ways. There is, for example, evidence showing that in novel hosts Wolbachia evolves rapidly and tends to gradually lose its virulence. If the lost virulence was to result in a decrease in their pathogen defensive qualities, the mosquito, having lost the efficiency of its conventional immune defences, could end up being more vulnerable to infection than before the Wolbachia introduction. Martinez et al.'s paper is a nice example of how investigating the evolutionary potential of such Wolbachia-host-pathogen interactions can be hugely informative as to the long term prospects of these new control methods.
Reference
[1] Martinez J, Cogni R, Cao C, Smith S, Illingworth CJR & Jiggins FM. 2016. Addicted? Reduced host resistance in populations with defensive symbionts. Proceedings of the Royal Society of London B 283:20160778. doi: 10.1098/rspb.2016.0778
Review: 1

An interaction between cancer progression and social environment in Drosophila
Cancer and loneliness in Drosophila
Recommended by Ana Rivero based on reviews by Ana Rivero and Silvie HuijbenDrosophila flies may not be perceived as a quintessentially social animal, particularly when compared to their eusocial hymenopteran cousins. Although they have no parental care, division of labour or subfertile caste, fruit flies nevertheless exhibit an array of social phenotypes that are potentially comparable to those of their highly social relatives. In the wild, Drosophila adults cluster around food resources where courtship, mating activity and oviposition occur. Recent work has shown not only that social interactions in these clusters condition many aspects of the behaviour and physiology of the flies [1] but also, and perhaps more unexpectedly, that social isolation has a negative impact on their fitness [2].
Many studies in humans point to the role of social isolation as a source of stress that can induce and accelerate disease progression. The ultimate proof of the connection between social interaction and disease is however mired in confounding variables and alternative explanations so the subject, though crucial, remains controversial. With a series of elegant experiments using Drosophila flies that develop an inducible form of intestinal cancer, Dawson et al [3] show that cancer progresses more rapidly in flies maintained in isolation than in flies maintained with other cancerous flies. Further, cancerous flies kept with non-cancerous flies, fare just as badly as when kept alone. Their experiments suggest that this is due to the combined effect of healthy flies avoiding contact with cancerous flies (even though this is a non-contagious disease), and of cancerous flies having higher quality interactions with other cancerous flies than with healthy ones. Perceived isolation is therefore as pernicious as real isolation when it comes to cancer progression in these flies. Like all good research, this study opens up as many questions as it answers, in particular the why and wherefores of the flies’ extraordinary social behaviour in the face of disease.
References
[1] Camiletti AL and Thompson GJ. 2016. Drosophila as a genetically tractable model for social insect behavior. Frontiers in Ecology and Evolution, 4: 40. doi: 10.3389/fevo.2016.00040
[2] Ruan H and Wu C-F. 2008. Social interaction-mediated lifespan extension of Drosophila Cu/Zn superoxide dismutase mutants. Proceedings of the National Academy of Sciences, USA, 105: 7506-7510. doi: 10.1073/pnas.0711127105
[3] Dawson E, Bailly T, Dos Santos J, Moreno C, Devilliers M, Maroni B, Sueur C, Casali A, Ujvari B, Thomas F, Montagne J, Mery F. 2017. An interaction between cancer progression and social environment in Drosophila. BiorXiv, 143560, ver. 3 of 19th September 2017. doi: 10.1101/143560